Ara Hacobian Ph.D. Head of Molecular Biology Ludwig Boltzmann Institute for Experimental and Clinical Traumatology
David Hercher Ph.D. Master of Science Ludwig Boltzmann Institute for Experimental and Clinical Traumatology
Improving Efficacy of Therapeutic DNA Vectors
Gene therapy represents a potent therapeutical application for regenerative medicine. So far, viral and nonviral approaches suffer from major drawbacks hindering efficient gene therapeutic applicability: the immunogenicity of viral systems on the one hand, and the low gene transfer efficiency of nonviral systems on the other hand. Therefore, there is a high demand for improvements of therapeutical systems at several levels. This review summarizes different DNA vector modifications to enhance biological efficacy and efficiency of therapeutical vectors, aiming for low toxicity, high specificity, and biological efficacy—the cornerstones for successful translation of gene therapy into the clinic. We aim to provide a step-by-step instruction to optimize their vectors to achieve the desired outcome of gene therapy. Our review provides the means to either construct a potent gene therapeutic vector de novo or to specifically address a bottleneck in the chain of events mandatory for therapeutic success. Although most of the introduced techniques can be translated into different areas, this review primarily addresses improvements for applications in transient gene therapy in the field of tissue engineering.
Introduction
Gene transfer, a method to introduce exogenous genes into host cells, represents the state-of-the-art technology to evaluate functions and expression patterns of genes. Furthermore, it is used for immunization and as a potential therapeutic for gene replacement therapy in various inherited or acquired diseases. Gene therapy represents the delivery of therapeutic factors as genetic information encoded on a DNA vector and has also become a potent tool in tissue engineering and regenerative medicine. Improvements in the design of novel gene delivery agents have already led to several effective in vitro, in vivo, and ex vivo techniques, including growth factor delivery, short interfering RNA delivery, and gene replacement therapies.
Compared to the delivery of recombinant proteins, gene therapy provides several advantages such as lower manufacturing costs and increased bioactivity of the produced proteins due to host-specific posttranslational modifications and correct folding of the locally produced growth factors. Furthermore, a sustained release of therapeutic factors is often favorable in terms of regenerative capacity especially in the field of musculoskeletal diseases and injuries.1 Opposed to this, a burst effect that takes place after application of recombinant proteins often results in unwanted adverse effects.
To introduce exogenous DNA into cells in vitro and in vivo, several strategies based on viral or nonviral approaches are available.2,3 Viral gene transfer strategies [retroviruses,4 adenoviruses,5 adeno-associated viruses,6,7 herpes viruses,8lentiviruses,9 and Epstein–Barr viruses (EBV)10] show by far the highest transfection efficiencies, whereas nonviral vectors are limited in their efficacy to deliver genes. Nonetheless, the application of nonviral gene delivery systems in vivo is gaining more popularity due to the disadvantages associated with viral systems such as detrimental immune responses and chromosomal integration.11 Furthermore, nonviral vectors have low manufacturing costs, are easy to handle, and provide transient expression kinetics of therapeutic factors, of special interest in the field of regenerative medicine.12,13Nevertheless, independently of used gene delivery strategy, optimized and improved DNA vectors are essential and will boost the promising field of gene therapy further to the clinics. This review summarizes different DNA vector modifications to enhance biological efficacy and efficiency of therapeutical vectors (Figures 1 and 2). The following methods and tools for gene therapeutic vectors are not restricted to a specific type of therapeutical system or application and hence can be adapted to improve both viral and nonviral vectors.
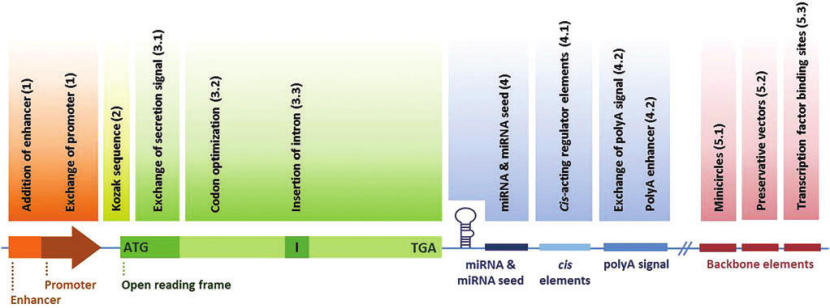
Figure 1. Schematic overview of sequence regions that can be modified to improve efficacy of vectors. miRNA, microRNA; polyA signal, polyadenylation signal.
Promoter/Enhancer
Many constitutively active promoters are used to induce overexpression of exogenous genes (Table 1). Among them, the cytomegalovirus (CMV) promoter (basal promoter in combination with its enhancer region) is one of the best studied promoters for mammalian target cells.14–16 It has been demonstrated to be active in a wide range of cell types and tissues and is one of the most commonly used promoter for gene therapeutical approaches.15,17,18 Special emphasis should be put on its upstream enhancer (USE) sequence harboring additional binding sites for several transcription factors.15 The enhancer element can be combined with other basal promoters to strongly boost the activity for constitutive17,19–21 and cell-type-specific22,23 promoters. Due to its viral origin, the CMV promoter itself is prone to silencing by methylation, resulting in a rapid decline of gene expression especially in stem cells.24 The potency of these cells as key players in regenerative processes makes it desirable to attain durable and high expression of exogenous genes at different developmental and differential stages.
To find the ideal driving force for gene expression, several studies have compared strength and duration of various promoters to drive gene expression across several cell types.19,24–32 Importantly, various cellular and viral promoters display distinct activity and expression kinetics during different cell stages. Among them, human elongation factor-1 alpha (EF1α) promoter drives reliable and high gene expression in stem cells and stem-like cells where other promoters (such as CMV) have reduced activity or are targets of silencing, especially in early developmental stages.24,31,33
Initial observations were made by Chung et al.24 in mouse embryonic stem cells, which were transfected with reporter genes under the control of different constitutive promoters. They showed that both the chicken β-actin (CAG) promoter and EF1α promoter showed high induction of reporter gene expression, while the CMV promoter was mainly inactive, becoming operative only after the stem cells were differentiated into neuronal precursor cells. Comparable observations were reported by another group, highlighting the inferiority of the CMV promoter in distinct cell types such as mesenchymal stem cells.32Similarly to the CMV promoter, the mouse phosphoglycerate kinase 1 promoter has also shown to be ineffective to induce high transgene expression in embryonic stem cells at early stages.33
Overall, EF1α shows the most universal and highest expression throughout the performed studies, followed by the CAG promoter. The large size of the latter may, however, be problematic for some applications.24,32
As a general issue, the selection of a promoter is commonly based on availability, rather than its suitability for a certain setup. This results in unused possibilities to improve therapeutical efficiency.
Notably, some studies have shown that specific genomic elements can be effective in preventing promoter silencing of viral promoters or enhancers. The efficiency of nonviral in vivo transfection of liver cells with a vector harboring the complete α-1-antitrypsin (hAAT) gene with its native promoter was evaluated.34 This DNA vector showed long-term expression of the therapeutic protein hAAT in mouse plasma (4 months), while DNA vectors without the 0.5 kb upstream element of the hAAT gene sequence (complementary DNA) showed a different expression profile with protein expression decreasing around 100-fold within 30 days.

Table 1. Overview of the Most Commonly Used Constitutively Active Promoters Used for Gene Therapy in Mammals
To read this article in its entirety or access its references click here.



