November 15, 2015 (Vol. 35, No. 20)
Life Sciences Researchers Talk About the Obvious Solution—Cell-Line Authentication—but They Fail To Implement It
According to a 2013 report from the American Association for the Advancement of Science, $115 billion is spent annually in the United States on life science research. Fifty percent of this total is spent on preclinical research, half of which—$28 billion—is not reproducible.
Irreproducibility causes delays and failures in clinical trials, drug development, and basic life science projects. A common contributor to this significant waste of research resources is the widespread use of misidentified or contaminated cell lines, which are estimated to be the source of more than one-third of all cells used in research.
In April 2015, the Global Biological Standards Institute (GBSI) launched a multifaceted campaign to raise awareness of the importance of cell-line authentication in improving research reproducibility. Besides suggesting that the research community needed to undergo a cultural change to begin taking cell-line authentication seriously, the GBSI issued a number of specific recommendations:
- Use standards and best practices: Documented authentic, contaminant-free cell lines should be used in research.
- Establish dedicated funding: Research grants should include the costs to address cell-line authentication.
- Authenticate to publish: Journals should require documentation of cell-line authentication.
- Commit to train: Graduate students and postdoctoral fellows should receive more information on the importance of cell-line authentication.
- Invest in new technologies: There should be greater investment in the development of novel tools for cell-line authentication.
In June 2015, the GBSI conducted a survey on authentication and found generally very poor compliance and suboptimal awareness of its importance. Most respondents indicated that they believed in the value of authentication, yet very few had ever done it themselves.
The top three respondent-identified barriers to performing cell-line authentication were cost (61%), time (53%), and delays (35%). More troubling is the apparent complacency surrounding the need to authenticate cell-lines: 24% of respondents reported not seeing the necessity of authentication, and a similar percentage indicated that management was unaware of or deliberately ignored the issue.
“We have an ISO/ANSI-accepted standard, which is rare in preclinical research, and we have contract laboratories that conduct the test at low cost. Yet, ironically, compliance is still abysmally low,” notes GBSI president Leonard Freedman, Ph.D. “That is where the GBSI focuses, not on new testing methodology.
The GBSI has been brainstorming approaches to convincing journals and funding agencies not to stop at encouraging authentication, but to proceed to requiring it, thereby mitigating, to some degree, what Dr. Freedman calls an irreproducibility crisis. “Principal investigators will not authenticate unless there’s a carrot and a stick,” Dr. Freedman insists. “But there are tremendous opportunities, both scientifically and financially—huge amounts of funds potentially saved—if the majority of scientists authenticated their cell-lines.”
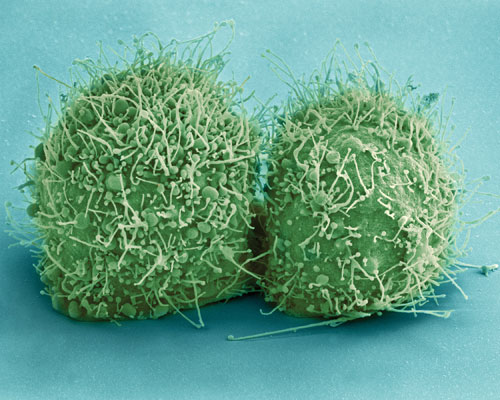
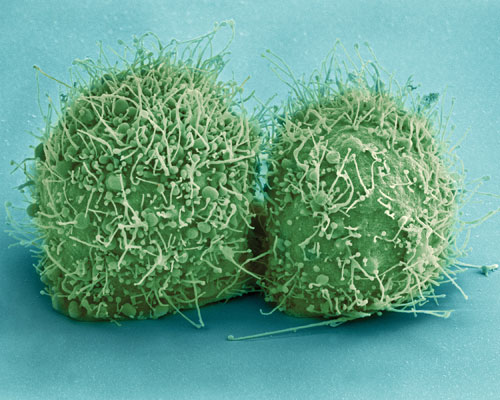
The HeLa cell line, represented here by a scanning electron micrograph showing a pair of recently divided HeLa cells, is the most common source of human cell-line contamination and misidentification. To minimize the chances that HeLa or other cell lines will cause cross-contamination problems—and potentially give rise to spurious results—researchers are encouraged to follow “best practices” recommendations, such as those issued by the Global Biological Standards Institute. This organization indicates that 36% of the cell lines being used today are not authentic.[National Institutes of Health (NIH)]
Everyone Wins
For Gabriela Saldanha, strategic marketing manager at Promega, the leading driver for cell-line authentication today is the National Institutes of Health (NIH), whose new guidelines for grant submission include the need to demonstrate that all proposed cell-lines have been authenticated.
NIH notice NOT-OD-15-103, Enhancing Reproducibility through Rigor and Transparency, includes provisions recommending demonstration of cell-line authentication from all investigators applying for NIH grants. The notice’s guidelines, which become effective January 16, 2016, were discussed September 28–29 by various stakeholders at the NIH Workshop on Reproducibility and Cell Culture Studies.
“That’s big,” Saldanha tells GEN. “For years, there has been talk about authentication, but honestly it was wishy-washy. Of all the concerns about reproducibility in science, cell-line authentication is one of the most important steps.”
But with the time and costs involved in authentication, some research groups are bound to object, are they not? Won’t authentication requirements divide winners and losers? Saldanha disagrees: “Everybody’s a winner. Everyone benefits by enhancing the quality of science.”
Scientists, however, may benefit more than others. “They will enjoy peace of mind,” she allows. They will have confidence that their cell lines “are what they think they are.” Also, best-in-class companies may come to regard automatically authenticated cell-lines as a relatively cheap form of insurance. Multimillion dollar research projects will be less likely to fail because they omitted what should be an automatic exercise.
“The benefits far outweigh any concerns I’ve heard or read about,” Saldanha adds. “Companies need only find a reputable service vendor, perhaps at an external core facility. Promega can help as well. We can provide examples of reports they can use, recommend how to follow ANSI guidelines for authentication, and explain how to perform the matching probability calculation, how to match their cell line to a database.”
According to Saldanha, the short tandem repeat (STR) assay, which is recommended by ANSI guidelines and remains the gold standard, is no longer expensive. Even authenticating at the beginning, middle, and end of a project will add only a few hundred dollars to project costs. When was the last time, Saldanha asks, anyone has paid less than that for the simplest piece of equipment?
For its part, Promega offers STR kits for fingerprinting cell-line DNA. The company, informs Saldanha, supplies reagents to service providers, core facilities, cell banks, and paternity labs.
Genomic Analysis
The most recent trend in cell-line authentication is the move to DNA sequencing techniques over earlier methods based on phenotypes. In the biopharmaceutical industry, mammalian cell-line identification and characterization testing has long used isoenzyme analysis, a phenotypic, four- to seven-enzyme method to identify mammalian cell-lines at the species level. But the recent and sudden limitations on the commercial availability of isoenzyme analysis kits have driven companies to seek out alternative identification methods.
“While sophisticated phenotype-based systems exist for in-depth metabolic analyses of cell-lines, simple and rapid authentication options now rely on genomic DNA sequencing—DNA profiling—for cell-line identification,” says Debbie Letham, Ph.D., senior scientist, biologics testing solutions, Charles River Laboratories. “The protocols are in place, but now there is an increased urgency to perform these alternative assays to fill the gap for customers.”
A DNA-based assay of particular interest is the analysis of a “barcode” region, a short stretch of unique DNA within the cytochrome oxidase I (COI) gene. Variations within this region are conserved, but enough differences are harbored between species to achieve species-level discrimination.
“Within a species, there may be minimal nucleotide differences,” Dr. Letham adds, “But compared to other species, even closely related species, there are more remarkable differences.”
The use of the COI region for identification purposes has been the mission of an international collaboration, the Consortium for the Barcode of Life (CBOL). This group has compiled a public library of COI region sequences known as the Barcode of Life database (BOLD). Multi-locus analyses, such as STR and multi-locus sequence analyses, are also very beneficial to subspecies (strain) characterization.
Although regulations strongly recommend a minimum of a top-level organism identification, prudent scientists gather as much information as possible for the cell-lines they bank on to control the production process of pharmaceutical end-products.
“Multimodal testing provides increased confidence that the cell substrates used to produce biological products are fully characterized, reducing potential risk in your process,” Dr. Letham explains. “Deep whole-genome analysis for sample identity or sample integrity is becoming less expensive, and therefore its use for fully characterizing strains is expanding.
Dr. Letham notes that cell-line authentication is important for identifying bacterial, fungal, and insect cell hosts that produce recombinant pharmaceuticals: “The same characterization rules apply for these as well as mammalian cell-lines.”
Microbial identification is also useful for trending environmental contaminant monitoring. Simple yet powerful comparative DNA sequence-based identification tools are widely used, such as Charles River’s AccuGENX-ID® for identification of the 16S rRNA gene in bacteria and the ITS2 rRNA region in fungi. Additionally, microbial identification utilizing proteotypic MALDI-TOF mass spectrometry technology provides a unique protein spectral fingerprint.
Standards for Nonhuman Lines
Among the attendees at the September NIH Workshop was Yvonne Reid, Ph.D., manager/scientist, ATCC (American Type Culture Collection). For human cell-line identity, the ATCC has published a consensus standard based on STR technology, which was adapted from forensics applications and is now being used broadly by cell banks, core labs, and others.
According to Dr. Reid, no validated assay equivalent to STR exists for authenticating nonhuman cell-lines. However, very promising, up-and-coming technologies, such as STR profiling of mouse strains under development by Jamie Almeida at NIST, is a good start.
“We have published on methods based on cytochrome C oxidase 1 barcoding technology for interspecies identification—mouse from rat from dog, for example,” Dr. Reid explains. “We are working on a standard specifically for that application, where we can amplify relevant regions of the genome, sequence them, and check them for database matches to determine the exact species.”
The requirements for developing a standard for nonhuman cell authentication are robustness, accessibility, and ability to appeal to a large proportion of scientists. A standard meeting these requirements, Dr. Reid says, will enable meaningful comparisons of data, “where everyone is using the same technology.”
Dr. Reid hopes that the most-cited impediments to universal cell-line authentication—time and cost—will be addressed by new consensus standards. “There must be a change in attitude on the importance of authentication. The prices for tests at this point should not deter anyone; the quality of science should be the driver.”
The call for cell-line authentication began as a whisper. While it has not yet risen to a roar, leading researchers, journals, and funding agencies are joining the push to urge principal investigators to do the right thing. “I keep a folder in my desk, and in there is a document where I was quoted saying, in 2004, that cell-line authentication was important,” Saldanha relates. “I’m thrilled that after all these years many people agree with me.”