December 1, 2012 (Vol. 32, No. 21)
Tools for Effective Validation and Utilization
Gene silencing by RNA interference has become a key tool in research and drug discovery since its discovery by Andrew Fire and Craig Mello. RNAi allows for the sequence-specific knockdown of target genes with a subsequent phenotypic analysis, representing a straightforward method for ascribing functions to genes.
The advantages of RNAi include the high efficiency of the gene knockdown, the ability to easily target the gene of interest, as well as stable and long-term silencing by expressing shRNAs. This makes for a powerful tool that has been successfully applied to answer many questions in cell biology. There are also risks, however, when using RNAi that need to be considered carefully.
Researchers have demonstrated that RNAi sequences do not only bind to one target. These changes in the gene expression pattern of the cell and potentially of the phenotype give rise to an off-target signature. The unidentified effects of such a signature bear a high risk to create false-positive outcomes that can bring a complete project into jeopardy. Furthermore, even most up to date algorithm-based sequence designs show a knockdown efficiency that is generally at 80% or less.
The effects of such specific but low knockdown can be masked by the off-target signature with phenotypic changes being undetectable. These issues can make RNAi unpredictable, slow, and risky, in particular in drug discovery, where speed and reliability of results are crucial factors. Sirion Biotech has developed technologies that help overcome the drawbacks of RNA interference.
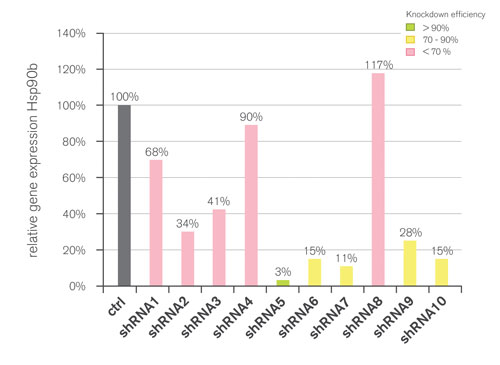
Figure 1. Screening of 10 shRNA sequences (shRNA1-10) for the silencing of Hsp90b: The shRNA5 was identified with a knockdown efficiency of over 95%. Sequence efficiency was confirmed on both mRNA and protein level in NIH-3T3 cells that were transduced with an adenoviral vector expressing shRNA5.
Screening for Active shRNAs
One particular challenge with shRNAs is finding sequences that are highly effective with a validated efficiency of over 80%. Sirion has developed RNAiONE™ for fast and reliable identification of the most efficient sequence for a specific gene.
RNAiONE has been used in the silencing of murine Hsp90b, a difficult target due to its abundance. Ten validation vectors containing different bioinformatically evaluated shRNA sequences targeting Hsp90b were transfected in an optimized cell system expressing the GFP-tagged Hsp90b protein from the same vector.
Initial microscopic analysis provided first evidence of shRNA efficiency based on the GFP readout. Validation of the knockdown was then carried out by measuring the mRNA level with real-time PCR and confirmed by Western blot analysis. The results show that the efficiency can vary tremendously for different target sequences with a knockdown between 0 and over 90% (Figure 1), demonstrating the need for an appropriate validation protocol.
The data also illustrates that a highly effective sequence could be successfully identified, despite the high abundance of Hsp90b in the cell. Furthermore, results with RNAiONE can be applied reliably to Sirion’s different viral vector platforms providing immediate access to subsequent gene analysis and assay cell model generation. The overall effectiveness of RNAiONE allows Sirion to identify shRNA for most of the genes and to issue warranties to its customer labs.
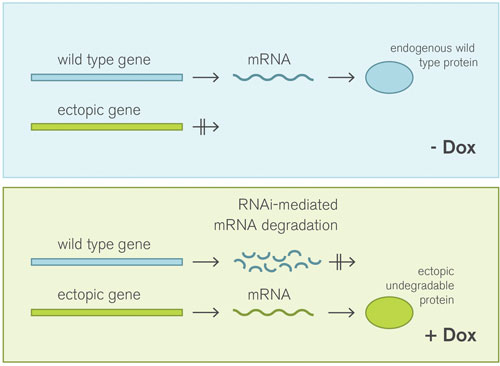
Figure 2. VariCHECK enables a simultaneous switch between a wild-type protein and the ectopic undegradable protein by simply adding Doxycycline into the cell media.
A Cell Model with RNAi Built-in Control
A prerequisite to successfully conduct meaningful shRNA experiments, however, is not only a high and validated knockdown, but also the use of appropriate controls. VariCHECK™ serves as such a perfect built-in control for RNAi experiments which allows confirmation of on-target phenotypes. This system enables the depletion of an endogenous targetX, while simultaneously overexpressing the ectopic undegradable form of targetX, which then rescues a specific phenotype (Figure 2).
The basis of VariCHECK is Sirion’s All-In ONE vector inducible lentiviral system based on Clontech’s latest 3G technology. The constitutive TET-dependent transactivator is simultaneously expressed together with the TET-inducible gene of interest or the validated shRNA. Cells are transduced with a first vector that contains the inducible shRNA targeting the wild-type gene of interest (GOI) and a second vector with the inducible ectopic undegradable form of the GOI.
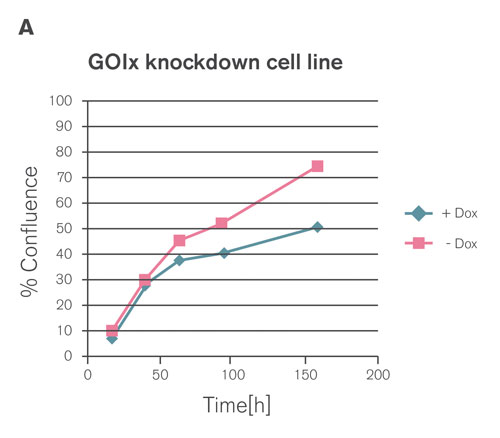
Figure 3A. VariCHECK serves as a perfect built-in control for RNAi experiments. Depletion of the oncology target GOIx significantly reduces cell proliferation in A549 cells.
Without Doxycycline (Dox), neither shRNA nor ectopic GOI expression takes place and the endogenous wild-type protein is present in the cell. In contrast, with Dox, wild-type transcripts are degraded by the expressed shRNA, while the ectopic protein is present.
The validity of VariCHECK has been successfully demonstrated for the functional analysis of an oncology target (GOIx). A stable cell line was generated that expressed the TET-inducible shRNA against GOIx, previously validated with RNAiONE. Gene knockdown was quantified, and the best performer cell clone with near quantitative knockdown was then transduced with lentiviral vector 2 that encoded the inducible ectopic undegradable GOIx.
Expression of GOIx in the absence and in the presence of Dox was quantified by real-time PCR and Western blot analysis, respectively, confirming an almost quantitative switch from wild type to an ectopically expressed form of GOIx on mRNA as well as protein level. Moreover, the inhibitory effect of GOIx knockdown on cell proliferation was fully rescued by ectopic expression of the undegradable GOIx, demonstrating the reduced proliferation as a real on-target phenotype (Figures 3A–C).
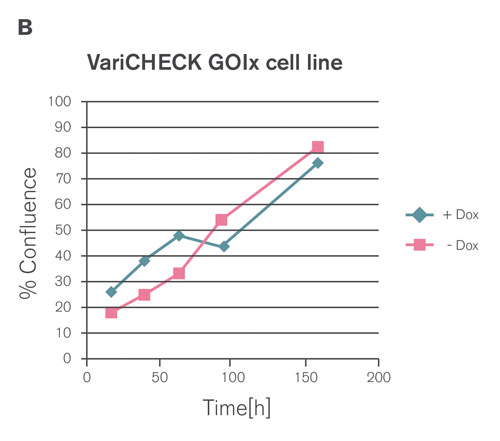
Figure 3B. Simultaneous overexpression of the ectopic undegradable GOIx fully restored proliferation, confirming a real on-target phenotype.
Beside its function as RNAi built-in control, VariCHECK can be also applied to reversibly switch expression between the wild-type protein and a defined mutant version in a single cell line. This approach can be used to undertake functional analysis of proteins and their mutations in a fast and equally reliable manner, which is of particular interest for the analysis of escape mutants from cancer drugs.
Since its discovery in 1998, RNAi silencing has become the method of choice for phenotype studies; however, there are some risks that need to be considered including an insufficient knockdown and potential off-target effects. This can be highly critical in drug discovery and development, and add another question mark in compound and target validation, a process that is already lengthy and risky. Technologies such as RNAiONE and VariCHECK make the use of RNAi for scientists faster, more predictive and reliable in particular in drug discovery.
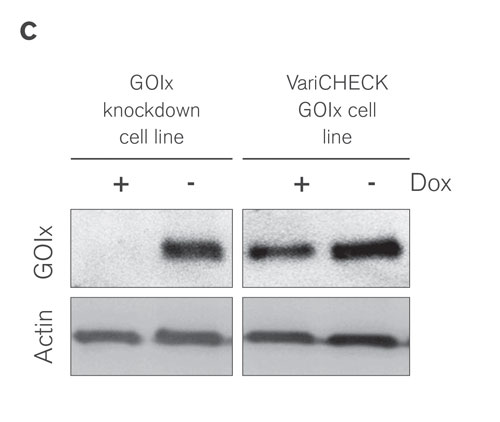
Figure 3C. Western blot confirms the near quantitative switch from wild type to ectopic expression of GOIx after Dox treatment.
Kathrin Schmitt, Ph.D. ([email protected]), is director, key accounts of Sirion Biotech.