Traumatic brain injury (TBI) is a significant public health issue worldwide and is predicted to be the third largest contributor to the global disease burden by 2020 1,2. The multifaceted and heterogeneous pathological aspects of this disease, which occur within days to months postinjury, cause significant neurological sequelae in TBI patients. Current empirical evidence provides new insight into these pathological mechanisms that lead to both focal neurological, as well as cognitive, deficits 3–5.
Recovery following TBI is complex and incompletely understood, yet studies have begun to elucidate important aspects of endogenously activated mechanisms that facilitate the process. Much of this research has been conducted to understand the fundamental concept of plasticity. Although neurogenesis within the mature brain continues, it is limited primarily to the subventricular zone (SVZ) surrounding the lateral ventricles and the subgranular zone (SGZ) of the hippocampal dentate gyrus (DG) 6–8. A distinct subpopulation of cells from these regions migrate through adult white matter and differentiate into neurons in several cortical locations. Recent evidence suggests these cells may be involved in cell repair or renewal mechanisms 9,10.
Exploitation of this endogenous population of stem cells is of particular interest with regard to TBI. Following both diffuse and focal injury, a significant increase in proliferation within the SVZ and DG has been demonstrated in both mouse and rat TBI models alike 11,12. Importantly, newly generated and injury-induced granular cells are able to integrate into the existing hippocampal circuitry, a phenomenon thought to facilitate innate cognitive recovery following injury 13,14. A more recent study of human TBI models found proliferation of cells expressing markers of neural stem cells (NSCs) and neural progenitor cells in the perilesion cortex, thus representing an intrinsic effort by the injured brain to repair and regenerate damaged tissue 15.
This observed endogenous plasticity can be further investigated and manipulated using precise electrical modulation. To date, several methods have been explored to induce or accelerate functional and adaptive recovery in TBI patients, including both invasive (eg, electrical cortical stimulation ECS) and noninvasive (eg, transcranial magnetic stimulation TMS, transcranial direct current stimulation, and pharmacologic) methods, each mediating an upregulation in plasticity following TBI 16–20. However, animal studies and clinical trials involving the use of these interventions are scarce, and such approaches are often cell type indiscriminate, invasive, and render surrounding tissues susceptible to damage 21,22. Due to a universal understanding that newer therapeutic approaches must circumvent these limitations, recent developments have successfully incorporated precision and cell type specificity into the treatment modality. Optogenetics builds upon previous research through the use of genetically encoded channels and receptors that serve to selectively activate or inhibit neuronal subpopulations with unprecedented spatial resolution and millisecond temporal precision. In this review, we discuss optogenetics as a means to evaluate and modulate neural circuits in the context of recovery following TBI.
Fundamentals of Optogenetics
Optogenetics is a modern advancement incorporating the fields of bioengineering, optics, and genetics for the purpose of modulating and monitoring cellular activity at the level of molecularly defined neuronal classes. This innovation involves the artificial introduction of light-sensitive proteins (eg, opsins) into cell membranes 23,24. Neuronal plasma membranes themselves are thus made sensitive to light, permitting direct activation and inhibition of specified, targeted neurons within intact neuronal circuits 25. In addition, optical monitoring of neuronal activity is achieved using genetically encoded sensors that respond to changes in ion concentration (eg, calcium) or membrane voltage. By utilizing tools with the ability to utilize light energy, neuronal imaging can achieve both high spatial and high temporal resolution 26,27.
While previous approaches typically fall short with respect to temporal and spatial accuracy, optogenetics expands the capability for optical imaging and genetic targeting by simultaneously controlling or monitoring either the activity of many neurons within a circuit or certain regions within a single neuron. Single-cell optogenetics is able to map neural circuits with excellent accuracy and zero-spike crosstalk 28. Expression of certain light-sensitive proteins can also behave as actuators and switch neurons on and off, inducing either depolarizations or hyperpolarizations for varying periods of time with exquisite precision. This capability allows the opsins to probe neural activity at the resolution of single spikes, raising the possibility that this method can one day mimic natural neural code 29. Since its inception, optogenetic tools have been developed to further map complex neural circuits and target specific neurons to facilitate behavior modulation, which are significant ambitions of current research in the field of neuroscience.
Overview of opsins
Opsins are a class of light-activated, seven-transmembrane proteins that are capable of undergoing conformational change to function as ion channels or sensory receptors. The proteins are classified as either microbial (type I) opsins or animal (type II) opsins 30–32. Type I opsins bind retinal in the all-trans configuration and are found in prokaryotes, algae, and fungi. In contrast, type II opsin genes encode G-protein-coupled receptors (GPCRs) used predominantly for vision, and thus bind retinal at low levels of light in an 11-cis configuration 32,33. Type I opsins demonstrate certain biochemical properties—such as a decreased chromophore turnover rate and lack of coupling to second-messenger cascades—that allow type I opsins to accelerate optical control of specialized cellular processes with faster on/off kinetics than type II opsins. As such, type I opsins have become a valuable option in the laboratory setting 32.
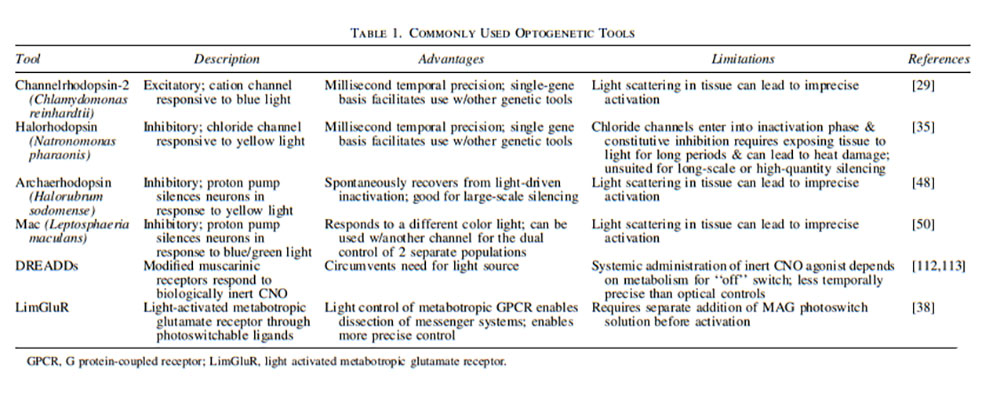
Optogenetic actuators
Channelrhodopsins (ChR1/2), originally identified in Chlamydomonas reinhardtii, a green alga, were the first microbial opsins described that were found to function both as photoreceptors and ion channels 29,34,35. This unique combination of receptor characteristics affords the opportunity to both control and record signaling dynamics that form the basis of neuronal circuitry using novel stimuli. For example, photoactivation of ChR2 can generate single action potentials within a selected neuron to map out specific synaptic connections within a circuit 29,36.
ChR2 was first used to manipulate neuronal activity over a decade ago; since then, there has been sufficient evidence collected to support the use of opsins in the recovery of functional connectivity in intact neural circuits in response to naturalistic spike trains or rhythmic activity 29. ChR2 demonstrates blue light-induced isomerization within 50 μs of exposure. The receptor opens its channel pore and the subsequent cation flux leads to depolarization and excitation of the particular neuron that expresses the receptor. This finding suggests that expression of ChR2 may be an effective tool to manipulate cytoplasmic calcium concentration or to depolarize the cell membrane 35. Expression of ChR2 has also been shown to optically activate neurons within the frontal cortex of primates with millisecond precision. In addition, while activation occurred in targeted locations containing excitatory neurons labeled with the receptor, light modulation of neural activity in fibers of passage was successfully avoided 37.
Light-responsive glutamate receptors are GPCRs that have also been developed using photosensitive ligands, and thus serve as a means of investigating endogenous synaptic transmission mechanisms 38,39. Metabotropic glutamate receptors (mGluRs) have a well-established role in the regulation of multiple cellular processes, including neuronal excitability, neurotransmitter release, and neuroplasticity, thereby justifying further examination into their use as an optogenetic target 40,41.
Additional optogenetic channels have been isolated that can permit further modulation through neuronal silencing. One such channel type is represented by Halorhodopsins (NpHR), derived from the bacterium Natronomonas pharaonis. NpHRs are chloride channels that generate ion flux following illumination with green/yellow light, thus inducing hyperpolarization and inhibition of target neurons 42–44. NpHR-induced silencing is both rapidly inducible and reversible, with recovery of normal activity observed within milliseconds of yellow light cessation 45. However, the rhodopsin has been found to exhibit slower recovery kinetics following extended illumination to yellow light due to the protein entering an inactive state. This delay in hyperpolarization resulted in the suggestion that the use of NpHRs be restricted to neuronal silencing of shorter durations 46,47. Subsequent research, however, found that supplementary exposure of NpHR to blue light for brief periods facilitated conversion of the rhodopsin back to its active state, thus promoting optimal performance of Halorhodopsin and silencing of the target neuron 45,47.
Similar activity is seen with another opsin from Halorubrum sodomense, archaerhodopsin (Arch). Arch is an outward proton pump that responds to yellow/green light 48. Unlike NpHRs and other light-driven chloride pumps that are limited by their poor recovery kinetics, Arch spontaneously recovers from its inactivation 48. Archaerhodopsin-3 (Arch-3) gene expression induced near 100% neural silencing in the awake mouse brain 48. Another archaerhodopsin, ArchT, isolated from Halorubrum strain TP009, also demonstrates effective neural quieting with fast kinetics. However, ArchT does so with improved light sensitivity leading to an increased brain tissue volume being influenced by the proton pump compared with Arch 48,49.
Isolated from the fungus, Leptosphaeria maculans, Mac represents yet another light-driven proton pump. It also enables neural silencing but does so by responding to green-blue light. Therefore, there is potential to stimulate neurons through ChR2 while simultaneously silencing neurons downstream through Mac—thus providing dual control of two separate populations 50. An overview of optogenetic tools can be found in Table 1.
 Type II opsins
Type II opsins
Although past research has focused on type I opsins, recent developments have proved type II opsins to be potential tools for optogenetic control. In 2009, Deisseroth et al. engineered a family of opsin-receptor chimaeras, termed “opto-XRs,” by replacing the intracellular loops of bovine rhodopsin with either Gq-coupled human α1a-adrenergic receptors or Gs-coupled hamster β2-adrenergic receptors, leading to IP3 or cAMP pathway activation, respectively 51. Without any exogenous retinoid supplementation, they detected significantly increased Ser 133-phosphorylated CREB (an indicator of proper signaling cascade function) in the illuminated optoXR-expressing population 51.
Transgenic introduction of opsins
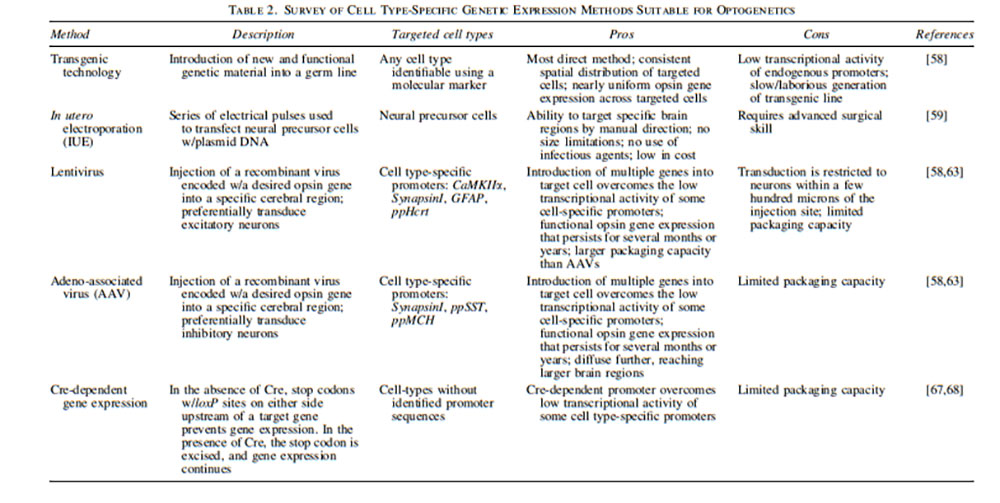
Several methods have been described for expressing opsins in neurons, each having its own profile of strengths and weaknesses regarding factors like cost, expression pattern, and procedural difficulty. The most straightforward method is represented by the transgenic expression of ChR1/2 in a variety of animal species (eg, zebrafish, fruit flies, mice, rats, and one species of roundworm, Caenorhabditis elegans)—with each opsin exhibiting receptor expression in distinct brain locations 52–56. Unfortunately, significant obstacles exist with strategies involving these transgenic animal models that complicate the process. For one, generation of a transgenic line is both slow and laborious. Additionally, inadequate opsin gene expression occurs due to the low transcriptional activity of endogenous promoters 57,58.
In utero electroporation represents another technique, whereby neural precursor cells are transfected with plasmid DNA by a series of electrical pulses, thus affording the ability to target specific brain regions with manual direction 59. This approach is low in cost and represents a safe method that avoids the use of infectious agents. One disadvantage, however, is that it requires advanced surgical skill and, therefore, leads to fewer animals suitable for experimentation 57,60,61.
The use of viral vectors is the most common approach, providing targeted expression when the use of transgenic technology is unsuitable 57,58. This process involves injection of a recombinant virus encoded with a desired opsin gene into a specific cerebral region 57. Introducing multiple genes into each target cell overcomes the low transcriptional activity of some cell-specific promoters and precipitates high levels of opsin gene expression 58. Lentiviruses and recombinant adeno-associated viruses (AAVs) are the most commonly used viral vectors. Transduction through these methods produces functional opsin gene expression that persists for several months 58,62,63. Lentiviruses are physically larger with a larger packaging capacity. However, because of this large size, transduction is restricted to neurons within a few hundred microns of the injection site. AAVs, however, are smaller and diffuse further, thus reaching larger brain regions 62. The vectors have also been shown to differ in preference for inhibitory or excitatory neuron populations when introduced into mouse somatosensory cortices, such that rAAVs preferentially transduce inhibitory neurons and lentiviruses have a preference toward excitatory neurons 63. Therefore, the endogenous tropism of the two vectors may be utilized to facilitate the isolated expression of opsin proteins in specific neuron populations.
Further targeting of specific cell populations can be achieved using various cell type-specific promoters. Such populations already elucidated as targets include vesicular γ-aminobutyric acid (GABA) transporter (VGAT), tyrosine hydroxylase (TH), tryptophan hydroxylase (TPH), choline acetyltransferase (ChAT), and myelin basic protein for GABAergic inhibitory, dopaminergic, serotonergic, cholinergic, and oligodendrocyte populations, respectively 64–66. When targeting cell types that lack identified promoters, Cre-Lox recombination can be used to induce gene expression 67. In 2016, Kawano et al. created a photoactivatable Cre-recombinase that demonstrated a 320-fold increase in DNA recombination in vivo 68. These results were upheld even with low-intensity or pulsed illumination 68. As a result of these findings, approaches for genetic introduction of opsins into neurons can be tailored to specific population subsets while generating high rates of successful gene expression. An overview of methods for genetic expression of opsins are displayed in Table 2.
 Optogenetic sensors
Optogenetic sensors
In addition to neuromodulation, optogenetic technology is used to monitor electrical dynamics within intact neuronal circuits. Genetically encoded voltage indicators (GEVIs) and genetically encoded calcium indicators allow for in vivo visualization and quantification of several physiological events with a high signal-to-noise ratio and the potential for single-cell resolution 69,70. Voltage-sensitive dyes are another commonly used tool with favorable kinetics, sensitivity, and spectral tuning. However, these dyes lack genetic targetability to a specified population of cells and are further restricted by phototoxicity 71,72. Optical methods have demonstrated marked improvement over other methods (eg, traditional electrophysiology) as well because of their ability to monitor neuronal circuitry with less invasive, better targeted, and superior multisite visualization 73. GEVIs are genetically engineered and light-emitting protein sensors used for sophisticated readout of neuronal voltage dynamics. By demonstrating enhanced voltage range specificities, the sensors allow for differentiation between axon depolarization, subthreshold potentials, and synaptic activity 69,74. In 2013, the ArcLight GEVI was found to exhibit a high signal-to-noise ratio. Optical recording by ArcLight was therefore able to describe the electrophysiological characteristics of each neuronal membrane compartment, namely the soma, neurites, and synaptic terminals 75.
An all-optical integration of neuronal circuits has been described to illustrate the combined effect of neuronal activity sensors and neuromodulatory agents that permits single-spike precision 76. Optical signals respond in millisecond time-scale of fast electrical signaling and are large enough to allow monitoring of voltage changes at the single-cell level 77.
Closed-loop optogenetics
Closed-loop optogenetics describes the process of basing optogenetic stimulation on simultaneously observed dynamics. As such, full optical control over neuronal activity is possible 78. When guiding stimulation in a nonclosed loop setting, inputs are not affected by measured output. In contrast, closed-loop control involves a time-varying light stimulation pattern that is automatically updated based on error-sensing negative feedback. The error signal is therefore the difference between measured and desired output trajectory 78. Closed-loop optogenetics could monitor and correct deviations within neural systems, thus permitting real-time control over neural dynamics and behavior 78.
The “Optopatch” is the closest approach toward “all-optical” integration. Coexpression of CheRiff, an optogenetic actuator protein, with the new QuasAr voltage indicators enables simultaneous and crosstalk-free optical perturbation and measurement with a high signal-to-noise ratio, resulting in delivery of spatially and temporally precise stimuli to neurons 76,79. Importantly, this development was the first to exhibit homeostatic plasticity of intrinsic excitability in human induced pluripotent stem cell-derived neurons 79.
Therapeutic Implications for TBI
Several endogenous mechanisms involved in TBI recovery have already been identified. An understanding of these processes, as well as the basic pathophysiology of TBI, remains fundamental when attempting to harness the innate restorative capacity of the human brain. These concepts are equally important in regard to the development of modern therapeutics to be used to alleviate chronic neurological dysfunction post-TBI.
TBI pathophysiology and potential targets for treatment
The course of TBI consists of two distinct phases, both of which serve as potential targets for neuromodulatory therapeutic approaches. The first phase follows the primary insult. Mechanical disruption from an external force results in the deformation of brain tissue, manifesting as damage to blood vessels and axonal shearing. As a result, diffuse neuronal depolarization occurs with subsequent release of excitatory neurotransmitters—including glutamate and aspartate—at both the site of injury and in surrounding tissue 80–82. This injury-induced process leads to substantial calcium influx, activating several downstream cellular processes 83,84. In contrast to the shorter primary phase of injury, secondary injury evolves over minutes to months. During this subsequent phase, a multifactorial set of biochemical cascades lead to apoptosis and formation of reactive oxygen species. Effects of the process result in ischemic and hypoxic damage, cerebral edema, increased intracranial pressure, brain atrophy, and ultimately, clinically relevant functional deficits or posttraumatic neurological disability 80,85. Moreover, mechanical insult is only the initial event of TBI. Overt clinical symptoms of this multifaceted process may not manifest for several years after the time of injury. Secondary, delayed injury can manifest through several molecular mechanisms that ultimately result in a number of pathologic changes, including altered cerebral blood flow, transient disruption of blood–brain barrier integrity, local and systemic inflammation, changes in oxygen delivery and metabolism, and both ischemic and apoptotic death of neural cells 86–89.
Intravascular clot formation is another common finding following TBI, potentially leading to local ischemia and systemic coagulopathy due to consumption of clotting factors 90,91. Additionally, the pathological increase in extracellular calcium concentration and resultant injury-induced neurotoxicity can generate a progression leading to circuit disruption or dysfunction, such as epileptogenesis 92,93. For example, the risk of developing epilepsy is found to be increased up to 10 years postinjury 94. This potential for a long latent period between initial insult and onset of symptoms provides a window during the clinical course for devising therapeutic intervention.
Potential targets for optogenetic stimulation include neurons that would benefit from prolonged cell survival post-TBI to preserve cognitive ability, as well as, targets that can attenuate seizures postinjury. Stimulating areas of the SVZ and SGZ of the DG has been shown to enhance cognitive recovery long term in mouse models 95. In addition, several areas of the brain have been shown to attenuate seizure activity when targeted with optogenetic therapeutics. These areas include the DG, cerebellum, medial entorhinal cortex, ventrobasal thalamus, motor cortex, and superior colliculus 49,96–101. To date, no non-neural cells have been found to be effectively modulated for treating TBI.
Optogenetics for the study and treatment of TBI
The use of optogenetics as a method to monitor brain tissue following TBI represents a new field in neuroscience, with only two studies detailing its use.
After inducing repeated mild TBI in mice expressing ChR2, Adams et al. measured neuronal function through bilateral intracranial electrophysiological recordings in response to optogenetic photostimulation. They found that this photostimulation resulted in reduced evoked neuronal response and neuronal functional deficits, in both pericontusional tissue and in tissue in the contralateral hemisphere 102. This study thus represents the first in-situ application of optogenetic photostimulation as a tool for TBI treatment and analysis.
In the same year, Zhang et al. developed another model to monitor the brain’s response to TBI. By combining nanotechnology and optogenetics, these authors developed a stretchable transparent electrode array using carbon nanotube (CNT) to record response signals from cortical surfaces following photostimulation. Their results indicated successful production of signals with high temporal resolution and negligible light-induced artifacts 103. The CNT electrodes were also shown to maintain functionality during and after brain contusion, thus enabling real-time electrophysiological monitoring of neural activity throughout the course of injury 103.
Neural stem cells
While transplantation of NSCs has been shown to mitigate cognitive and motor deficits after TBI in several studies, the mechanisms by which they do so remain unclear and have thus inhibited their optimization. Optogenetics can help elucidate how these grafted NSCs modulate neural circuits via enhanced NSC targeting. While studies have been conducted to demonstrate the combined effect of stem cell-based therapy and optogenetics in stroke models, few exist using TBI models.
To date, there has been only one study examining the potential use of neural stem cells in combination with optogenetics for TBI mapping and treatment. In this study, Zhao et al. used ChR2-EGFP (enhanced green fluorescence protein) coupled with doublecortin (DCX), a microtubule-associated protein expressed by neuronal progenitor and postmitotic neuronal precursor cells, to monitor and encourage the survival and maturation of nascent neuronal cells following TBI 95. To do this, a lentiviral vector carrying the DCX-ChR2-EGFP gene was constructed and injected into the hilus of the DG in C57BL/6 mice. After inducing TBI through a lateral fluid percussion protocol 104, optrodes were implanted into the DG of the injured hemisphere. The authors found that the number of EGFP-expressing cells began to rise and peaked at 3 and 9 days following TBI, respectively. Using this information, the authors selected day 3–12 post-TBI as the ideal time to perform in vivo optical depolarization of the ChR2 light-sensitive channels in these mice. Compared with the control group, these TBI+ChR2 mice demonstrated significantly greater performance on spatial learning and memory tasks. Furthermore, the TBI+ChR2 group showed increased maturation and migration of newborn neurons. At a molecular level, in vitro studies showed that optical stimulation specifically resulted in an upregulation of several neural marker transcripts, including microtubule-associated protein 2 (MAP2), neuronal nuclei (NeuN) antigen, Neurogenin 2 (Neurog2), neuronal differentiation 1 (NeuroD1), and glutamate receptor subunit 2 (GluR2) in newborn cells 95. Altogether, these findings suggest that this technique promoted neurogenesis following depolarization of stem cells expressing the opsin, thus providing evidence for its future implications in not only TBI but also many other neurological disorders.
Control of seizures in patients post–TBI
Optogenetics has greatly enhanced our ability to both research the mechanisms of seizures, as well as, treat seizures with optogenetic therapies 105. Targeting specific areas of the brain with opsins has been shown to ameliorate seizures at their early stages of initiation 98. Halorhodopsin has been well described as being able to attenuate seizure activity by targeting areas of the brain, such as the hippocampus, ventrobasal thalamus, motor cortex, and cerebellum 96–98. ChR2 has been shown to reduce seizures in several areas, such as the cerebellum, medial entorhinal cortex, superior colliculus, and hippocampus 99–101,106. These therapies could be used for patients after TBI that suffer from epilepsy as a result of their injury. Furthermore, it can be developed and used successfully in closed-loop systems that could measure output from the patient’s electrocorticogram or electroencephalogram to initiate therapy as appropriate. Closed-loop optogenetic mechanisms have already been successfully shown to measure seizure output and also treat absence seizures in mouse models 107.
Translational barriers and future directions for clinical use
While optogenetics offers a multitude of potential uses, challenges and possible translational barriers must be addressed—the most significant being the fact that light does not penetrate bone 108. As both photoactivatable proteins and optoXRs require direct in vivo illumination, invasive surgery, thermal activation, and phototoxicity thus limit their therapeutic capabilities 109,110. However, because of this, there has been research on methods to combat the phototoxic effects. Mostany et al., for example, developed a two-photon excitation (2PE) paradigm, which utilizes lower energy photons and fluorescence confined to the area of interest. This, in combination with the longer excitation wavelength required, and its ability to analyze signals from scattered emission photons, 2PE microscopy can serve as a safer imaging technique to be combined with optogenetics 111.
Chemogenetics is a neuromodulation method that represents a noninvasive alternative to optogenetics that requires minimal equipment and has potential for chronic activation. Like optogenetics, this technology also offers the enhanced spatiotemporal resolution of GPCR signaling 110,112. The newest of these tools, DREADDs (Designer Receptors Exclusively Activated by Designer Drugs) are engineered GPCRs that are selectively activated by a biologically inert compound, clozapine-N-oxide (CNO). Selective binding of CNO to DREADDs allows spatial control over neuronal excitation and inhibition, whereas its unique pharmacokinetics offers temporal control 112,113. In 2019, Chandrasekar et al. used DREADDs in mouse TBI models to increase the excitability of principal neurons in the acute phase following injury, thus resulting in activity-dependent survival 114.
Photoswitchable Orthogonal Remotely Tethered Ligands (PORTLs) are utilized in another photopharmacological approach. PORTLs contain a receptor ligand, namely glutamate, which photoisomerizes following exposure to specific wavelengths of light, thus permitting photoactivation of mGluRs 115. As mGluRs have been implicated in cellular processes following TBI, such as mechanisms that attenuate neuronal cell death, the receptors are a potential target for treatment 116,117. In 2013, Levitz et al. found that light-activated metabotropic glutamate receptor 2 (LimGluR2) utilizes photoswitchable ligands to switch on and off with millisecond precision. These fast effector kinetics therefore permit modulation of activity on a synaptically relevant timescale 38.
DREADDs, PORTLs, and Opto-XRs, all represent engineered GPCRs that can be turned on and off within genetically targeted cell types. However, these approaches require significant alteration of the receptor sequence. This may result in receptors that are unable to bind to their endogenous ligands, making their physiological roles obsolete 115. Newer methods have been developed to target endogenous receptors, therefore circumventing limitations associated to receptor sequence modification. Drug Affinity Responsive Target Stability technology, which provides membrane-anchored ligands that target endogenous receptors, has recently been combined with PORTLs to permit genetically specified and reversible GPCR activation 115. Although further investigation is required to assess the efficacy of such technology for TBI treatment in both animal and human models, these methods represent potential alternatives that offer advantages over current treatment modalities.
As for the future clinical impact of optogenetic modulation, the authors believe it will, one day, have the potential to offer minor therapeutic benefit in patients post-TBI. As both TMS and ECS currently have limitations to their use, optogenetic modulation can be used to stimulate survival of neurons in addition to offering an accurate method of measuring postinjury changes in the patient’s brain. Deep-brain stimulation (DBS), to date, has limited but promising evidence for having a therapeutic benefit in TBI patients, and optogenetic modulation could be used in addition to DBS 118. There is also early data that photomodulation, which uses red/near-infrared light therapy to prolong survival of neurons, improves cognition in patients long term after TBI with little negative side effects, and optogenetics could also supplement this therapy as well 119. As for the most effective method of introducing opsins into patients, the most promising methods seem to be by genetic transduction with both lentiviral and AAV vectors, as each different vector has different tropisms and can target different types of neural cells in a variety of different areas of the brain. These transductions will further supplement the transplantation of genetically modified cells such NSCs and mesenchymal stromal cells, and these genetically modified cells are also being continually revised and improved to optimize functional prognosis post-TBI 120,121. To date, there seems to be no clear advantage between either the transduction of opsins or transplantation of genetically modified cells, as both have limited evidence to measure their effectiveness in human patients.
Conclusion
Although TBI remains a leading cause of disability worldwide, postacute treatment options are lacking as recovery following such injuries is complex and multifactorial. Several potential therapies are being studied, but few have provided evidence of therapeutic potency in human trial. With the high spatiotemporal precision provided by optogenetics, unique pathophysiological mechanisms of TBI may be identified and targeted for therapy.
Please visit Stem Cells and Development‘s special issue Stem Cell Therapy for Brain Injury to access the references and to read this article in its entirety.
Stem Cells and Development, published by Mary Ann Liebert, Inc., is globally recognized as the trusted source for critical, even controversial coverage of emerging hypotheses and novel findings. The above article was first published in Stem Cells and Development‘s February 2020 issue. The views expressed here are those of the authors and are not necessarily those of Stem Cells and Development, Mary Ann Liebert, Inc., publishers, or their affiliates. No endorsement of any entity or technology is implied.