August 1, 2018 (Vol. 38, No. 14)
Pioneering CRISPR Researchers Guide New Startup Companies along Divergent Paths
CRISPR startups, like all high-tech startups, climb Mount Commercialization faster if they get an extra boost, which may come from a unique technology, a compelling application, or a hoard of venture capital. Some CRISPR startups, however, benefit from another sort of boost—the presence and guiding intelligence of a true CRISPR pioneer.
Although CRISPR pioneers are few—names such as Jennifer Doudna, Feng Zhang, and George Church come to mind—they accomplish so much that they seem to be in several places at once, directing expansive research agendas here, and participating in diverse commercial ventures there.
Four to five years ago, CRISPR pioneers helped launch the first group of CRISPR ventures, most notably Intellia Therapeutics, CRISPR Therapeutics, and Editas Medicine. As these names indicate, the first CRISPR ventures started by emphasizing therapeutic applications. In contrast, newer CRISPR ventures are striking out in multiple directions. Besides exploring therapeutics, the new startups, many of which are also associated with CRISPR pioneers, are looking into diagnostics, agriculture, and other application areas.
For some time now, the CRISPR pioneers have been on each other’s heels. Even before joining the first group of CRISPR ventures, these scientists were publishing the breakthrough papers that gave CRISPR a new meaning. Initially, CRISPR (clustered regularly interspaced short palindromic repeats) emerged as a genomic pattern emblematic of bacterial immune systems. Then, thanks to the CRISPR pioneers, CRISPR came to stand for bacterial immune system elements that, upon reengineering, may serve as powerful gene editing tools. For example, several CRISPR-associated (Cas) nucleases have emerged, each endowed with distinctive capabilities.
Currently, the CRISPR pioneers are starting new ascents of Mount Commercialization. Rising from their base camps, the CRISPR pioneers may soon be on each other’s heels again—or not, if they follow different paths. There is, as this article illustrates, a lot of ground to cover.
Mammoth Biosciences
One new company researching and developing CRISPR applications in the therapeutic space is Mammoth Biosciences, headquartered in San Francisco, CA. Notable researcher and CRISPR pioneer, Jennifer Doudna, Ph.D., serves as both the co-founder and chair of the company’s scientific advisory board.
According to Mammoth’s CEO and co-founder, Trevor Martin, Ph.D., Mammoth was created to democratize diagnostics that rely on genetic information. “Right now, there are a lot of trends in medicine, especially in personalized medicine and tailored therapies,” says Dr. Martin. “In general, we think the industry is shifting toward early and improved diagnostics.”
The researchers at Mammoth are searching for novel functions of CRISPR that go beyond identifying viruses, bacteria, and disease-related genetic mutations. For these researchers, the ultimate goal is the creation of an in-home genetic testing device that can provide point-of-care disease detection. These researchers assert that the company’s device, which is still in its prototype phase, will retain design elements that are meant to support economy and user friendliness.
“At Mammoth, we have the world’s most affordable and easy-to-use programmable system for performing diagnostics at the molecular level,” Dr. Martin insists. Although Mammoth’s CRISPR-based platform has yet to enter routine clinical practice, the company anticipates that its technology will revolutionize the way diagnostic tests are performed.
Instead of going to the doctor’s office, patients can use Mammoth’s technology to identify health issues at home, thereby improving healthcare access and reducing the time spent traveling to and from testing facilities. Patients using the technology start by placing a fluid sample on a paper strip. Then the patient uses a smartphone to take a picture of the paper strip to capture any color change that may occur. Next, the patient uploads the picture to Mammoth’s “companion app,” which interprets the image and generates a test result. According to Mammoth, the wait for a test result lasts just 30 minutes.
“One of the reasons we’re really excited about this platform is its programmability,” Dr. Martin declares. “It’s always the same protein we’re using in every test, and with that we’re actually switching out the guide RNA.” An additional feature of the Mammoth platform, according to Dr. Martin, is its ability to detect the presence of a pathogen as well as identify the detected pathogen’s specific strain.
Beam Therapeutics
Another CRISPR pioneer, Feng Zhang, Ph.D., of the Broad Institute of MIT, is credited with co-founding Beam Therapeutics, a company whose primary focus is on base editing for altering sequences of DNA. This new precision medicine company recently secured $87 million in Series A funding from F-Prime Capital Partners and ARCH Venture Partners. Using these funds, Beam hopes to focus on base-editing platforms. Such platforms enable the direct, irreversible conversion of one base pair to another at a target genomic locus.
Currently, Beam has licensing agreements for two base-editing platforms developed by Harvard researcher David Liu, Ph.D. One platform is a C base editor, which delivers programmable C-to-T or G-to-A edits in DNA. The other platform is an A base editor, which delivers programmable A-to-G or T-to-C edits.
Additionally, the company licenses an RNA editor platform developed in Dr. Zhang’s laboratory. Beam did not comment on its plans for this platform when it was contacted by GEN, but since it emerged from stealth mode, the company indicated that it will develop a pipeline of precision genetic therapies that repair disease-causing point mutations.
Pairwise Plants
In early 2018, Monsanto (now a part of Bayer) invested $125 million in Pairwise Plants, a startup cofounded by Dr. Zhang, Dr. Liu, and fellow CRISPR stalwart Keith Joung, Ph.D. The company was built around the idea of CRISPR technology, specifically base editing technology, to deliver agricultural gene editing services.
Monsanto’s gene editing strategy lead, Scott Knight, Ph.D., tells GEN that the Pairwise-Monsanto venture was intended to enable the biotech giant to accelerate the use of gene editing in the top five agricultural plants: corn, soy, cotton, canola, and wheat. Pairwise-Monsanto’s plans included the development of plants possessing the ability to resist disease and tolerate drought or flood, as well as the ability to produce flavorful food.
CRISPR is exciting because it gives developers the ability to go into the genome to create new variation. “But to use the tool effectively,” Dr. Knight advises, “you have to do the work beforehand and afterwards to really understand the underlying basic biology and genomics.” Only then, he suggests, can developers use CRISPR to leverage the natural genetic diversity in agricultural crops and accelerate plant breeding.
“Separate and apart from our work in row crops, we are exploring gene editing applications for a wide variety of items you see every day in the produce aisle,” states Tom Adams, Ph.D., CEO of Pairwise. Until recently, gene editing has been primarily involved in eliminating traits, yet Pairwise is working, Dr. Adams asserts, toward “enabling more subtle changes like modifying a single amino acid to enable new combinations of natural variants.”
Arbor Biotechnologies
In addition to participating in Beam Therapeutics and Pairwise Plants, Dr. Zhang is contributing his expertise to Arbor Biotechnologies, a CRISPR startup that counts him among its co-founders. This New England–based early-stage life sciences organization garnered approximately $15.6 million in Series A funding back in June 2017.
In a recent issue of the journal Molecular Cell, the company’s researchers reported the discovery of a new CRISPR enzyme, Cas13d. This protein, which is smaller than other Cas proteins, may be more easily packed into the viral vectors that are used to deliver CRISPR components. Arbor’s discovery platform, which incorporates genome sequencing and artificial intelligence technologies, is credited with discovering the enzyme. Using the platform, Arbor’s research team hopes to continue its identification and optimization of CRISPR proteins for constructing innovative biotechnology applications.
CasZyme
CasZyme, a CRISPR startup based in Vilnius, Lithuania, boasts a relationship with another CRISPR pioneer: Virginius Šikšnys, Ph.D., one of the first researchers to show that CRISPR-Cas9 can be “programmed” to create double-strand breaks in specific DNA sequences. Dr. Šikšnys founded CasZyme and serves as the company’s chair.
CasZyme, like other CRISPR startups, embraces collaborations with other biotechnology firms. A partnership between CasZyme and New England Biolabs is focused on identifying and commercializing CRISPR-Cas nucleases. In another partnership, CasZyme enjoys access to DuPont Pioneer’s CRISPR-Cas library. CasZyme is applying its biochemical assays and expertise to characterize the Cas nucleases. DuPont Pioneer intends to use the nucleases to advance its plant breeding efforts, which are aimed at the development of seed products that have greater environmental resiliency, productivity, and sustainability.
Locus Biosciences
Locus Biosciences was founded three years ago to pursue healthcare-related CRISPR applications, primarily precision antimicrobials. The company is particularly focused on using the Cas3 helicase/nuclease. Unlike Cas9, which surgically cuts double-stranded DNA, Cas3 “shreds” single strands of DNA that have been separated from their matching strands.
Cas3 works with a riboprotein complex known as Cascade. When Cascade recognizes foreign DNA, it binds to a target sequence and causes double-stranded DNA to form a loop, which attracts the attention of Cas3. Joining Cascade and seizing the loop, Cas3 reels in the target DNA, separates its strands, and cuts one of the strands repeatedly along its length while, at the same time, continuing to yank more DNA through the Cascade-Cas3 machinery.
Cas3, which is far more prevalent than is Cas9 in bacteria and archaea, may be exploited, Locus asserts, to therapeutic effect. Essentially, Cas3 can induce a pathogen’s CRISPR bacterial immune system to attack the pathogen itself. While Cas3 may one day find use as a precision gene editing tool, it has, to date, been of interest mainly for its antimicrobial potential. For example, Cas3 may prove valuable in countering antibiotic-resistant strains of bacteria.
Locus’ chief technology officer, Dave Ousterout, Ph.D., emphasizes that currently, Cas3 therapeutic relevance depends on the enzyme’s ability to damage targeted DNA beyond repair. “Cas3 behaves as a unique single-stranded exonuclease,” he says. “It binds DNA and then rapidly degrades a single strand up to 1,000s of base pairs away from where it originally targeted the DNA.”
“There are no known systems that can efficiently repair DNA lesions caused by Cas3,” he continues. “Thus, every targeting event results in the killing of that cell.” In contrast, Cas9 produces clean double-strand breaks that are repaired by DNA repair mechanisms in bacteria, which ultimately allow for the survival of the cell.
At Locus, the hope is to create therapeutics that could replace antibiotics. To put this hope to the test, the company is moving toward a clinical trial with the help of approximately $19 million in funding that was secured in 2017. Currently, Locus researchers are using CRISPR to develop a new drug for complicated urinary tract infections caused by Escherichia coli bacteria, with a specific focus on the multidrug-resistant E. coli strains. Two other drugs following close behind include targeted therapies for Pseudomonas aeruginosa and Clostridium difficile.
eGenesis
The Boston-based eGenesis is a CRISPR startup co-founded by another genome engineering pioneer, George M. Church, Ph.D., a geneticist, molecular engineer, and chemist who holds senior academic posts at Harvard Medical School, MIT, and the Wyss Institute. By leveraging gene editing technologies, eGenesis is working to regularize xenotransplantation in medicine. The company’s ambition, the eGenesis website declares, is the delivery of “safe and effective human transplantable cells, tissues, and organs to the hundreds of thousands of patients worldwide who are in dire need.”
In March 2017, the company raised approximately $38 million and devoted part of its funding toward developing CRISPR-Cas9 technology that can be used to resolve a key xenotransplantation challenge: the inactivation of porcine endogenous retroviruses (PERVs). Because they can infect humans, PERVs have, to date, complicated the development of safer transplant-friendly pig organs.
“By leveraging CRISPR technology, eGenesis has the tools to tackle the cross-species viral transmission concern in xenotransplantation that was difficult to address before,” Luhan Yang, Ph.D., chief scientific officer and co-founder of eGenesis, tells GEN. Last year, eGenesis scientists reported that they not only used CRISPR-Cas9 to inactivate all of the PERVs in a porcine primary cell line, they also generated PERV-inactivated pigs via somatic cell nuclear transfer.
These achievements, according to a paper written by eGenesis scientists and published in Science, opens the door to a safer source of organs and tissues for pig-to-human xenotransplantation. “The team has created more than 30 pigs that have been born PERV-free,” she adds. “Today, these piglets are likely the most advanced genome-modified animals living on Earth.”
Realizing the Dream
Kunwoo Lee, Ph.D.
Two years ago, I had the opportunity to meet Roelof Botha, a prominent Silicon Valley venture capitalist, and describe the gene editing–delivery technology I’d been working on during my Ph.D. as well as its potential applications. After hearing me out, he asked: “How different will the life of a human being be decades from now because of this technology?”
I thought about this for a minute. “It will be a really different world,” I replied.
My CRISPR story begins in 2012. While I was a graduate student at UC Berkeley and UCSF, word spread of a new protein with the ability to precisely cut DNA sequences. Soon after, Jennifer Doudna, Emmanuelle Charpentier, Feng Zhang, George Church, and Jin-Soo Kim became CRISPR pioneers, publishing a series of groundbreaking papers about this novel technology. Their research inspired me to believe that gene editing has the potential to change medicine by curing genetic disorders. With the support of Doudna and Stanley Qi, my colleagues and I initiated a research project developing a delivery system for the CRISPR-Cas9 ribonucleoprotein.
Upon completing my Ph.D., I had two different career paths in front of me. One was academia, which was my dream entering graduate school. The other was the industrial route, working to enhance CRISPR-based gene therapies through the development of a unique delivery system. A voice deep inside my heart inspired me to take the road where I could make the biggest impact on people’s lives. Envisioning a future where CRISPR-based therapeutics could cure disease, I embarked on a journey of biotechnology, with the aim of revolutionizing gene therapy.
Launching a Company
In 2016, my co-founders Hyo Min Park, Niren Murthy, and I launched GenEdit, with the goal of creating the next generation of gene editing–based therapeutics. We set our vision, built a scientific team, and pitched our idea on how to shape the future of medicine. It is pure excitement to develop our technology and realize the company’s potential.
At the same time, we also encountered many of the inherent challenges that come with launching a startup company: financing, management, and responsibility. Convincing investors to believe in our vision required translating our scientific concepts into the language of business, including financial returns. I have endured many sleepless nights debating the direction of GenEdit. Velocity matters: high velocity means not only high speed but also the right direction. As CEO, I must decide the right course, which can be a lonely position at times.
My advice to other young entrepreneurs is to learn from your mentors. Every meeting and interaction I have is a chance to be taught something new. I am fortunate to have many opportunities to meet with entrepreneurs and pharma executives who have kindly shared their experiences, offered valuable input, and helped me find direction.
On the technical front, delivery of CRISPR to a specific target tissue is an unsolved challenge. Our focus is polymer nanoparticle technology. Polymers are advantageous in that they can deliver a protein form of CRISPR-Cas, unlike viral delivery or lipid nanoparticles. Moreover, various polymer structures have a unique interaction with cellular receptors and serum proteins, which are important parts of targeted delivery. In order to screen for the right polymer nanoparticle for each target tissue, we have synthesized a library of polymer nanoparticles with different sizes, charges, and targeting molecules.
Our initial results have been encouraging, as polymer nanoparticles showed efficient delivery of the Cas9 protein and guide RNA in muscle and neural tissues with direct injection. With the support of many superb academic collaborators, our talented scientists have delivered novel polymer nanoparticle systems that work in a variety of animal models, including Duchenne muscular dystrophy and fragile X syndrome (Figure).
Our recent publications illustrate that nonviral gene editing induces behavioral or functional changes in disease animal models.
Our next objective is to screen for systemically injectable nanoparticles. We are pushing the boundaries of in vivo gene editing and aim to move into the clinic in the near future.
A look back at the history of RNA interference, reveals that a huge amount of engineering work was conducted to achieve clinical translation. Even though CRISPR has already proven its potential in many preclinical studies, the CRISPR field is still in its infancy. Therefore, another innovation I anticipate in this field is the engineering of CRISPR systems, such as enhancement of the guide RNA and Cas9 protein.
Additionally, donor DNA is a necessary component if a genetic disorder requires precise gene correction by homology-directed repair. Multiple components need to work in an orchestrated way to guarantee the highest performance of CRISPR-based therapeutics. This is a field that basic and translational science needs to explore further; we are taking a systematic approach with an engineering mindset.
My experience has taught me that there are many paths to being an innovator. As Steve Jobs said, “We are here to put a dent in the universe.”
With my team at GenEdit, I aim to put a dent in next-generation medicine by transforming CRISPR technology to realize the dream and future of gene editing.
Kunwoo Lee, Ph.D. ([email protected]), is CEO and co-founder of GenEdit.
*Reprinted with permission of The CRISPR Journal, published by Mary Ann Liebert, Inc., Vol. 1, No. 3, 2018.
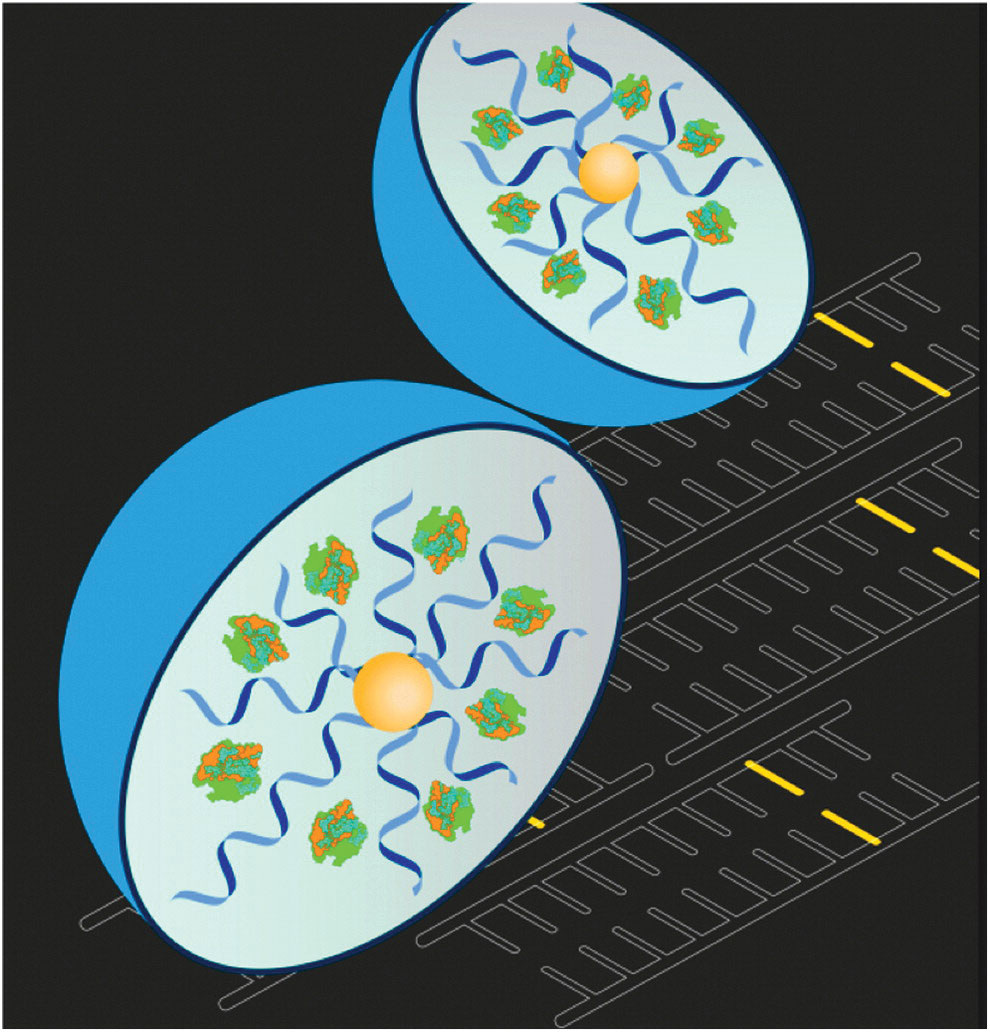
Polymer nanoparticles can deliver CRISPR protein and guide RNA to specific tissues.



