Neurodegenerative diseases represent the greatest therapeutic challenge of our time. Worldwide, an estimated 60 million people suffer from neurodegenerative diseases, which impair the function of the brain and central nervous system, and the number is expected to double every 20 years as the average age of the world population increases. These diseases, which include Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis (ALS), are devastating not only to patients, but also to caregivers, causing adverse personal and economic consequences that ripple throughout society.
For most neurodegenerative diseases, there are no approved cures, and palliative disease management has not improved in the last 30 years. The leading experts believe, however, that like cardiovascular diseases 25 years ago, and cancer 15 years ago, neurology is reaching a tipping point. A critical mass of new biological hypotheses and radically different developmental approaches will accumulate, and the result will be an explosion of new therapies to stop or slow the progression of neurodegenerative diseases.
A ferment in brain toxicity models
“The lack of effective treatments for neurodegenerative disorders stems from a scarcity of novel drug targets and a poor understanding of disease biology,” says Richard Peters, MD, PhD, president, CEO, and director of Yumanity Therapeutics. “There is still a lot we do not know about the brain.”
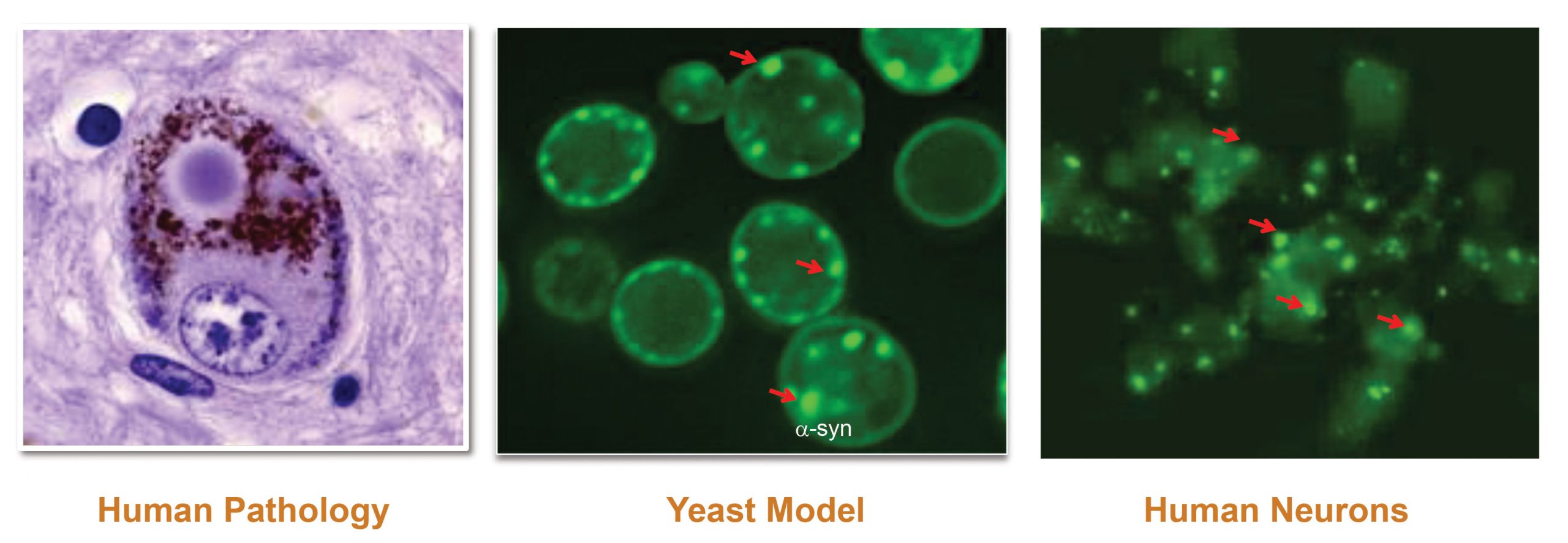
Yumanity’s unique approach underscores the need for creative solutions when it comes to diseases of the nervous system. Instead of matching chemistry to a known pathway, Yumanity pursues phenotype rescue in an unbiased screen. When the company’s founder, Susan L. Lindquist, PhD, then a professor at MIT, discovered that yeast can be used to model neurocellular pathologies, the next logical step was to identify chemical compounds that could reverse yeast responses to toxic neurodegenerative proteins.
“Yeast are a clever model to explore brain toxicity,” declares Peters. “Abnormal clumping or misfolding of proteins is evolutionary conserved. Be it yeast or human neurons, the responses to the toxicity are very similar. We have also discovered a correlation between the capacity of a compound to penetrate yeast membrane and its ability to traverse the blood-brain barrier.”
Peters indicates that Yumanity has identified other challenges with drug development for neurodegenerative diseases. Once the neurons have been damaged, reversing whatever degenerative processes have set in is unlikely. Therefore, the maximum therapeutic effect is achieved when the disease is diagnosed at early stages. Moreover, patients with the same diagnosis display the disease symptoms in a variety of ways. Such heterogeneity of population dilutes the treatment effect.
“To tackle hard-to-treat neurodegenerative diseases,” insists Peters, “the industry needs to understand and accept these challenges—and to structure their discovery programs accordingly.”
Yumanity (which is a play on words “yeast” and “humanity”) was able to move from target identification to the first human dosing with its leading brain-penetrating small molecule in just three years. The company’s lead product inhibits stearoyl-CoA-desaturase (SCD), an enzyme that catalyzes the synthesis of monounsaturated fatty acids (MUFAs). α-Synuclein, a hallmark of misfolded protein diseases such as Parkinson’s, is believed to bind MUFAs, creating protein inclusions and disrupting membrane biology and intracellular signaling.
Autologous stem cells sound a cord
“Enrichment is the hottest trend in neurodegenerative disease space,” asserts Ralph Kern, MD, chief operating officer and chief medical officer, Brainstorm Cell Therapeutics. “The oncology field was drastically transformed by utilizing genetic insights. In the neurosciences, we can generate a world of analytics from just a few drops of cerebrospinal fluid.”
Success in ALS clinical trials has remained elusive, with only a couple of new treatments approved in the past decade. Kern is certain that this phenomenon is largely due to the considerable heterogeneity in ALS symptoms. To overcome this problem, Brainstorm invests in biomarkers of cerebrospinal fluid to gain insights into disease mechanisms and to optimize patient stratification.
The company’s lead product for ALS, NurOwn®, contains injectable autologous mesenchymal stem cells (MSCs) that have been differentiated in vitro and stimulated to produce neurotrophic factors (NTFs). NTFs are known to promote the survival, repair, and regeneration of neurons. NurOwn® is injected directly into the spinal fluid, bringing it in immediate contact with affected motor neurons in the spinal cord. Changes in NTFs, inflammatory biomarkers, and biomarkers of neurodegenerative damage can be assessed directly from the same port.
The Phase II data demonstrated reduction in rate of ALS disease progression in the NurOwn treatment group. Cerebrospinal fluid biomarkers confirmed the secretion of NTFs and a reduction in inflammatory activity in the patients treated with NurOwn. The treatment effect was statistically significant in a subpopulation of participants with more rapid disease progression.
Kern predicts that the regulatory agencies will have to change the way they shape the drug development process, shifting from a preoccupation with common efficacy signals and large heterogeneous populations, to a willingness to favor unique efficacy signals and small homogeneous populations. “Signal readout is most easily observed in rapid progressors,” notes Kern. “[That is where] we can see meaningful slowing of the disease.”

He envisions that biological “software” solutions will become more prevalent, solutions that attempt to reprogram the disease environment by using the body’s own cells and repeatedly delivering new information. During Brainstorm’s manufacturing process, autologous MSCs are cryopreserved, with multiple doses of treatment being prepared from one bone marrow aspirate. This approach can generate multiple treatment doses while reducing the need for additional aspirates.
There’s a prescription app for that
Prescription digital therapeutics (PDTs) will experience explosive growth in the next few years,” predicts Yuri Maricich, MD, chief medical officer, Pear Therapeutics. “Our first FDA–market authorized therapeutic paved the way to other software innovations for treatment of many serious neurological diseases.”

Pear’s lead product, reSET®, is a 90-day PDT for the treatment of substance use disorder, which received de novo FDA classification as “computerized behavioral therapy.” It was soon followed by reSET-O®, an 84-day PDT for opioid use disorder intended to increase retention of patients in outpatient treatment. Digital therapy comprises a patient app and clinician dashboard and delivers cognitive behavioral therapy (CBT) to increase abstinence from substance use and increase retention in outpatient therapy programs.
“Partnership with clinicians is critical for the success of digital therapies,” asserts Maricich. “Our therapeutic provides additional treatment options that could be delivered anywhere, at any time, and it eliminates stigma that is sometimes associated with psychological interventions.”
Just like any molecular or cellular medicines, digital therapeutics are targeting scientifically validated mechanisms, and they are validated in randomized clinical trials. Maricich explains that neurodegenerative pathologies involve a significant behavioral component, which is often overlooked in pursuit of molecular interventions.
For example, fatigue, depression, and deficiencies in cognition continue to be of major concern for sufferers of multiple sclerosis even though this disease is reasonably managed by drugs targeting motor symptoms. “This is an example of significant unmet need and an opportunity for collaboration with existing molecular therapies,” Maricich points out.
reSET combines several widely accepted neurobehavioral approaches: an addiction-specific form of CBT known as the community reinforcement approach, fluency training, and a positive-reinforcement approach (contingency management).
In a randomized clinical trial, the study arm receiving reSET demonstrated a lower dropout rate and a greater abstinence rate. The software rewards a substance-free lifestyle, promotes skill building, and incentivizes abstinence. It also transmits information to the treating physician to assist in clinical decisions.
“Treatments for epilepsy, pain, and movement disorders are soon to be supplemented with digital therapies to enhance treatment efficacy,” ventures Maricich. “In a few years, the distinction between treatment modalities will dissipate.”
Keeping amyloid monomers under a cloud
“Until recently, the Alzheimer’s disease has been a graveyard of drug development,” states Martin Tolar, MD, PhD, founder, president, and CEO of Alzheon. “The Phase III results of Biogen’s therapeutic antibody aducanumab are cause for optimism because it has been confirmed that targeting amyloid can slow the clinical progression of Alzheimer’s.”
Tolar explains that amyloid in human brain occurs in three forms:
- “Good” amyloid monomers, which are important for brain health.
- Acutely neurotoxic “bad” soluble oligomers, toxic carriers in Alzheimer’s pathogenic cascade.
- Mostly “inert” fibrils and plaques, which sequester excess amyloid and protect against poligomer toxicity.
Selectively targeting “bad” amyloid oligomers may be a key to slow down or even prevent Alzheimer’s disease, as Alzheon has confirmed through its own contributions to amyloid research. The company’s lead product ALZ-801, a Phase III–ready novel prodrug of tramiprosate, inhibits the aggregation of monomers into oligomers.
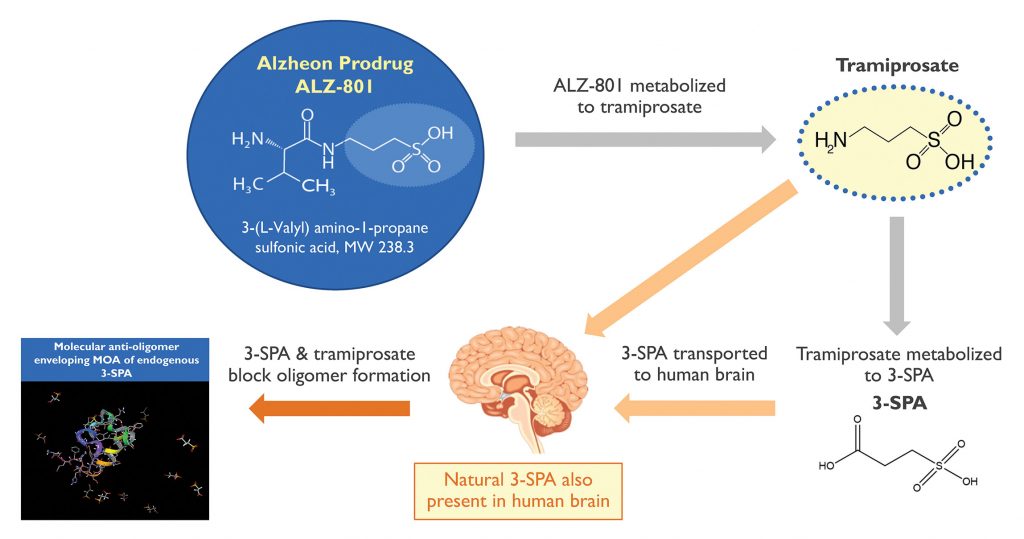
ALZ-801 is the first of a new class of therapies called “conformational modifiers.” The drug is transformed into an active metabolite, 3-sulfopropanoic acid (3-SPA), a molecule naturally found in human brain. Like ALZ-801/tramiprosate, 3-SPA stabilizes the conformational flexibility of amyloid monomers. When surrounded with several tramiprosate and 3-SPA molecules, the monomers adopt a shape that blocks their assembly into toxic oligomers.
“We determined that oral administration of tramiprosate significantly boosts the levels of 3-SPA in the brain and inhibits formation of amyloid oligomers,” continues Tolar. With a strong safety profile, high tolerability, and significant efficacy signals in APOE4 carriers, tramiprosate was granted Fast Track designation by the FDA.
Tolar also supports the enrichment strategy. “We are learning that certain forms of APOE4 protein and amyloid form a dangerous association, and that the APOE4 carriers have high levels of amyloid oligomers in their brains,” he explains. “This may be why these individuals develop the clinical symptoms of Alzheimer’s on average 15 years earlier than the noncarriers.
“However, structural damage in some brain areas such as the hippocampus precedes clinical symptoms. Tramiprosate had shown strong signals of protecting hippocampus from volume loss, offering a future possibility for preventive use.”
Tolar imagines a time when individuals at risk of Alzheimer’s will be able to use Alzheon’s medicines to prevent onset of symptoms and maintain their independence and dignity for many years.