As far as scientists are concerned, medical marijuana is exciting because it contains compounds that stimulate the endocannabinoid system, an intricate cell-signaling network that regulates many physiological processes, including those involved in pain, memory, mood, appetite, stress, sleep, metabolism, immune function, and reproductive function. To help medical marijuana realize its many potentialities, scientists are working to better characterize medical marijuana products and how they may exert different effects in different people.
In many instances, medical marijuana—that is, the marijuana that is taken for medical purposes—is basically the same as the marijuana that is taken recreationally. This kind of medical marijuana, then, shares recreational marijuana’s variability. The concentrations of the “active ingredients” can vary, complicating essential considerations such as dosing and whether a given formulation is suitable for a given condition. Also, it can be unclear how a formulation may perform differently in different people. Medical marijuana can also be understood to include drug products that are derived from (or inspired by) cannabis compounds. This kind of medical marijuana is typically better characterized and more consistent. Still, its effects can be variable.
These subtleties escape most cannabis retailers, and they can give medical professionals pause. Fortunately, help is on the way. Cannabis-oriented companies are bringing rigor to medical marijuana through genetic testing, formulation assistance, and drug development. Besides informing the cannabis industry and its consumers, these companies are supporting the work of the medical and pharmaceutical establishments.
Navigating the endocannabinoid system
Medical marijuana contains various compounds that can act on the endocannabinoid system in various ways in various circumstances, leading to various outcomes. All the possibilities can be overwhelming, especially since the endocannabinoid system is still, in many ways, terra incognita.
The endocannabinoid system was discovered in the 1990s by researchers studying the effects of tetrahydrocannabinol, or THC. Since then, studies have found that endocannabinoid receptors are located throughout the body. The primary receptor types are CB1 and CB2. CB1 receptors, which are located mostly in the central nervous system and brain, appear to serve as neuromodulators. CB2 receptors, which are expressed mainly in immune tissues, have been linked to immune system regulation.
These receptors are activated by naturally occurring endogenous cannabinoids, or endocannabinoids, made in the body. They can also be activated by compounds such as the cannabinoids, flavonoids, and terpenes made in plants.
Product matching based on genetics
Medical marijuana’s inconsistency is partly due to the variable nature of the cannabis plant and a lack of standards in processing and production. There are also many product types and consumption methods. In addition, medical marijuana consumers have different genetics and medical histories and take different medications.
As a result, first-time consumers often have no idea what effects, if any, they’re going to experience. They’re left trying multiple products or giving up on cannabis completely after going without the desired result or, worse yet, experiencing an adverse effect.
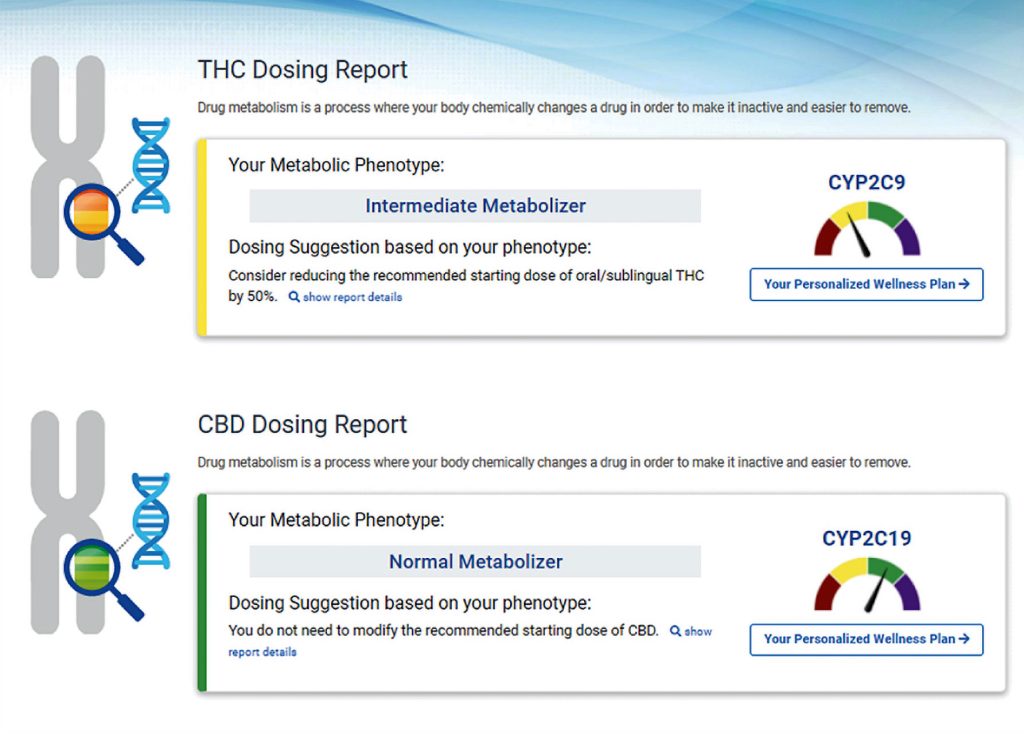
Endocanna Health offers a solution. The company provides a genetic test and cannabinoid formulation matching system to determine the product that will work best for each person and condition.
“In a nutshell, it looks at your 675,000 single nucleotide polymorphisms that are directly or indirectly associated with your endocannabinoid system,” says Len May, founder and CEO of Endocanna Health. “It gives you a full HIPAA-compliant portal report that is dynamic and is always updated based on new research.”
The system, which is called Endo-DNA, identifies an effective ratio of cannabinoids, the appropriate dose, and any potential drug interactions. “We line all that information up with our marketplace,” May adds. “We give you what the percentage of match is for that specific product based on your genetic predispositions.”
Finding an effective cannabinoid ratio
Some companies making cannabis products reduce the guesswork themselves. One such company is Zelira Therapeutics. It systematically evaluates how well its formulations perform for consumers, and it determines the optimal cannabinoid ratio. The company’s pipeline includes products for autism spectrum disorder.
The endocannabinoid system is responsible for the regulation of emotional responses, behavioral reactivity, and social interactions. And research has found that a deficiency of the endocannabinoid anandamide, which is related to the body’s stress response, is often seen in individuals with autism spectrum disorder.
Recognizing the connection, researchers have sought to treat the symptoms of autism spectrum disorder with cannabinoids and have reported some progress, especially in cases where THC was used in addition to cannabidiol, or CBD. Previously, when THC was used, it was present only in small amounts.
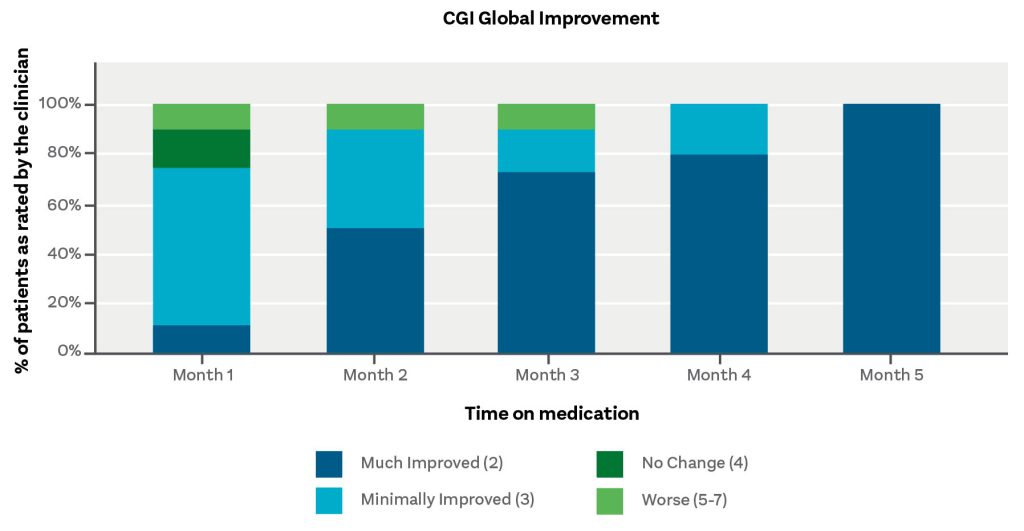
Zelira asserts that its research has demonstrated that a CBD:THC ratio of 1:1 has the most remarkable results in patients. Although the use of a psychoactive compound may give some parents pause, the Zelira CEO, Oludare Odumosu, PhD, suggests that they might be impressed by the encouraging results that Zelira has gathered for one of its products, HOPE 1.
Acording to Odumosu, an analysis of the first 45 patients in the study who used HOPE 1 for an average of 68 days showed a 68% reduction in irritability, a 55% reduction in aggression, and an almost 50% reduction in anxiety, while 60% of the participants reported a reduction in their meltdowns and almost 40% had an increase in focus.
“But even better, 69% of the parents believed that the quality of life for the child was improved,” Odumosu adds. “And 58% of the participants said they feel that they can report an improvement in the quality of life for their entire family.”
In addition to treatments for autism spectrum disorder, Zelira has developed treatments for insomnia and chronic pain. The company is also investigating cannabinoid-based treatments for a variety of cancers.
Chemical formulation using AI
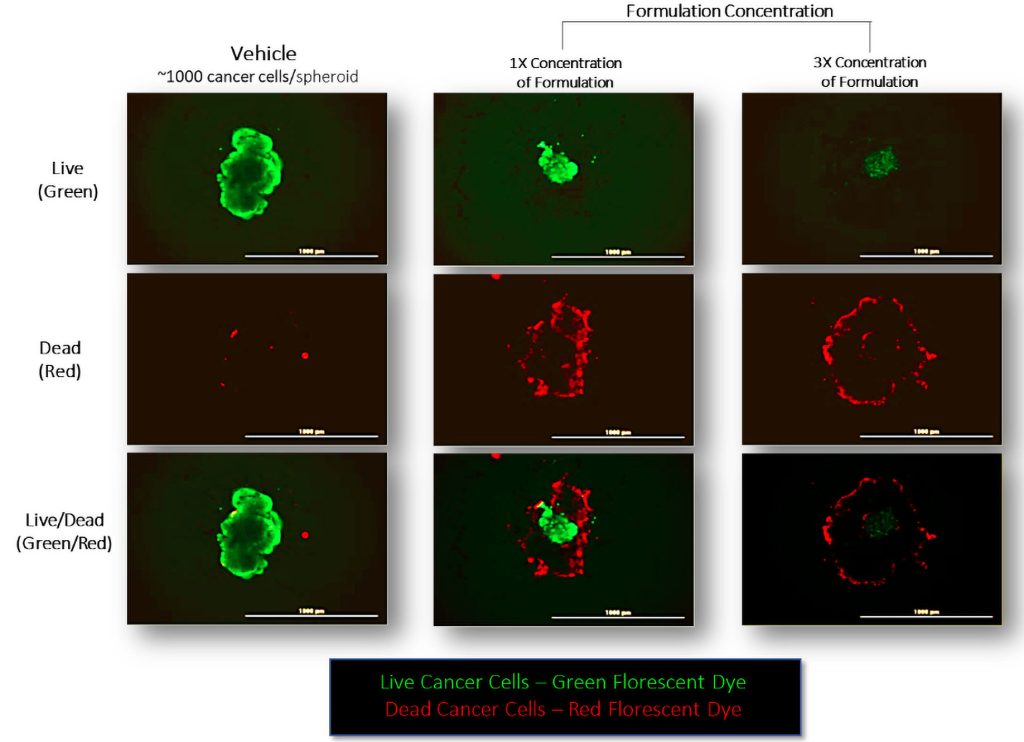
Cannabis compounds have been shown to induce cancer cell death through many pathways, including apoptosis pathways. These compounds are not necessarily limited to the familiar THC and CBD. To systematically determine which chemical compounds (and which combinations of compounds) would be effective in different circumstances would take a lot of resources.
Which is why Apollon Formularies used AI techniques to comb through a large database of cannabis strains to determine which specific combinations might best target cancer cells. Using this information, Apollon created a line of formulations specifically designed to kill cancer cells. The formulations were tested in preclinical studies in an independent, third-party laboratory using 3D tumor cultures of 10 different cancer cell lines. The results showed the concentration of each formulation needed to kill a large percentage of cancer cells for each cancer type.
Apollon is now combining cannabinoid-based cancer treatments with functional mushrooms, which have been shown to kill cancer through immune-stimulated cytotoxicity.
“Since Apollon’s AI platform can handle infinite dimensional space, we can analyze all of the compounds in cannabis and mushrooms together and take advantage of a ‘super entourage effect,’” says Stephen Barnhill, MD, chairman and CEO of Apollon. “We can create a cannabis-mushroom combination that is more effective than cannabis or mushrooms individually.”
An even more effective treatment can be identified when a patient’s genetic biomarkers are taken into consideration.
“Human genome sequencing data can also be analyzed through the same Apollon AI process,” Barnhill explains. “Doing so determines which biomarkers are important for triaging patients into the correct medical cannabis/functional mushroom treatment formulation for true personalized medicine, just as is currently done for traditional chemotherapy triage.”
Apollon’s cannabis formulations are available by prescription in Jamaica, where the company has a federally licensed production facility and a cancer institute for treating patients and performing clinical trials.
Mechanistic studies
Taking a closer look at the mechanisms behind cannabinoid-based cancer treatments is NKore BioTherapeutics. In a recent study, NKore co-founder Anahid Jewett, PhD, and colleagues studied the effects of a synthetic cannabinoid on cancer cells. The scientists compared the effects on cancer cells in well-differentiated tumors to those on cancer cells in poorly differentiated cancer cells.
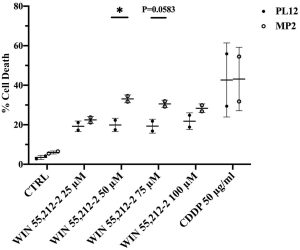
Since well-differentiated cancer cells have increased numbers of CB2 receptors, and since poorly differentiated cancer cells don’t have any on the surface, Jewett expected the cannabinoid to have much more of an effect on the well-differentiated tumors. What she found was just the opposite.
“My prediction did not come true,” said Jewett, who is also professor and director of the Tumor Immunology Laboratory at the University of California, Los Angeles. “We saw that these compounds were targeting more of the cancer stem-like/poorly differentiated tumors. That was very striking to us and a very important observation.”
Poorly differentiated tumors are more aggressive and harder to treat with existing chemotherapeutic or radiotherapeutic drugs. Currently, the most targeted and efficient way to kill these tumors is by the use of natural killer (NK) cells. Having another treatment option would be “very exciting,” Jewett insists.
There are still many questions that need to be addressed. One of the key questions concerns the targeting of cancer cells by cannabinoids. Jewett believes there may be a novel receptor that has yet to be identified on these tumors. Or they’re working through another mechanism entirely.
These unknowns highlight the current scarcity of cannabis research due to its classification as a Schedule I drug by the Drug Enforcement Agency and the resultant lack of federal funding.
“Schedule I means that cannabis is thought to have no therapeutic value and to have high abuse potential, says Swathi Varanasi, PharmD, co-founder and chief scientific officer for the CBD botanical wellness brand Element Apothec. “We now know that’s not true on either account. And so, there’s a huge movement now to get cannabis out of Schedule I.”
Other ways of targeting the endocannabinoid system
There are ways to activate the endocannabinoid system without relying on compounds found in cannabis. Many of the useful compounds found in cannabis plants are also found in other plants. And there are other plants have useful compounds that cannabis plants lack.
A useful compound that is present in cannabis and other plants is b-caryophyllene. This compound, which happens to be one of the most abundant terpenes found in black pepper, has been shown to activate the CB2 receptor, and studies have found that it could have a positive impact on the immune system.
Besides being stimulated by compounds found in plants, the endocannabinoid system may be stimulated by the endocannabinoids that are produced during activities such as exercise and meditation.
“One of the first biochemical processes that occur after you exercise is a rush of endocannabinoids and, in particular, anandamide,” Varanasi notes. “The feeling of the ‘runner’s high’ is caused by anandamide binding with the CB1 receptor, the same cannabinoid receptor for which THC has affinity.”
Compounds that behave like classical cannabinoids are known as cannabimimetics. According to Varanasi, they don’t receive enough attention.
“It’s bizarre that no one really talks about them,” she complains. “There’s not enough education out there, and the average healthcare professional doesn’t know about them because they’re not discussed in school. I think it’s really important to make them a part of the conversation.”


