January 1, 2012 (Vol. 32, No. 1)
CDI Offers Stem Cell Products for Studies on Heart, Liver, CNS, and Endothelial Tissue
Stem cells can be used for biological research and drug discovery, or to create new tissue and regenerate damaged organs. However, bans on the use of embryonic stem cells and the need for industrial quantities of high-quality stem cell lines slowed progress in the field.
Cellular Dynamics International (CDI) overcame these roadblocks by creating induced pluripotent stem cells (iPSCs), which are made from adult somatic cells like blood or skin.
CDI reprograms somatic cells into iPSCs through the incorporation of episomal vectors that convert cells to a stem cell state without modifying the genome. Because iPSCs are created from adult tissue, the company will never face a shortage of material, and they avoid the political controversy surrounding embryonic stem cells.
James Thomson, Ph.D., founded CDI in 2004 in Madison, Wisconsin. He recognized that highly purified iPSCs, and tissues differentiated from these lines, were needed in large quantities to spur stem cell research on. He started CDI with the goal of making such cells widely available and easy to use.
By December 2009, the company was making industrial-scale amounts of iCell® Cardiomyocytes, which exhibit the electrophysiological and biochemical properties of normal human heart cells. The iCell Cardiomyocytes not only reflect human biology, but they are manufactured and delivered in the quantity, quality, and purity required for drug discovery and toxicity testing.
CDI produces billions of iPSC-derived cardiomyocytes weekly, and customers like Roche use them to screen for potential cardiotoxicity of drug candidates. Researchers incorporate them into various applications including cardiotoxic compound screening, identification and investigation of arrhythmogenic compounds, and cardiac hypertrophy studies.
Roche and CDI began their collaboration in 2008 to explore the potential of iCell Cardiomyocytes to detect drug-induced, potentially life threatening side effects of drug candidates. The results were so successful that Roche now incorporates iCell Cardiomyocytes into its drug development decision-making process. In addition to supplying Roche, “our cells go to all the top 20 pharmaceutical companies,” says Chris Parker, chief commercial officer at CDI.
“Cardiomyocytes are now a catalog item that customers can buy,” says Bob Palay, CEO. The company’s other cell types, including neurons, hepatocytes, and endothelial cells, are available to customers under early-access agreements.
By supplying pharmaceutical companies with iPSC-derived, terminally differentiated cell types, CDI is pioneering an emerging service sector. The cells are shipped cryopreserved, then thawed and plated onto tissue culture plates with appropriate media.

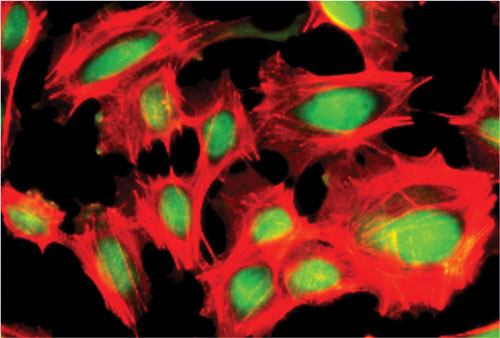
Immunostaining of iCell® Cardiomyocytes expressing the cardiac transcription factor Nkx2.5 (green) and F-actin (red)
Other Cell Types
The company’s iCell Neurons display dense axonal and dendritic processes and are a mix of GABAergic and glutamatergic subtypes. The firm notes that iCell Neurons provide a consistent and highly purified cell product for common discovery applications including high-content imaging, automated electrophysiology, and cell-based assays.
CDI is also developing iCell Hepatocytes. Liver toxicity is a common cause of preclinical failure during drug development, and drug-induced liver injury is one of the top reasons for withdrawal of approved drugs.
Current hepatocyte model systems include primary human cells harvested from cadavers, immortalized cell lines, and animal models, which are all limited in functionality, reproducibility, and availability. CDI’s iCell Hepatocytes overcome these problems and can be used to study hepatotoxicity, metabolism, cytochrome P450 induction, and transporter function.
Endothelial cells act as barriers between blood and body tissue, and they play a key role in inflammation and angiogenesis. The iCell Endothelial Cells in CDI’s pipeline can be used to study vascular diseases such as hypertension and cancer; delivery of neurological drugs across the blood-brain barrier; cell adhesion, invasion, migration, and proliferation; and tubular formation.
These other iCell products are undergoing beta testing by CDI’s early-access customers, who help to develop better products. “We work closely with customers and release the product only when we’re certain that it meets customer standards,” says Palay.
No matter what the iCell tissue type, “we have the power to produce cells in industrial quantities. That’s not trivial,” says Parker.
Researchers at CDI recently developed a method for making iPSCs using chemically defined media that’s free of feeder cells. The process is free of animal byproducts and sets the stage for manufacturing clinical-grade human iPSCs in a highly controlled GMP environment.
Such cell lines could be used for cellular therapy to repair damaged tissue or organs. “We’re setting the groundwork for potential long-term use of cells in a medical setting,” says Palay.
The company says it has also demonstrated for the first time that EBV-free iPSCs can be created from immortalized frozen samples stored in blood banks. The iPSCs retain their genotypic identity and differentiate into cell types representing all three germ layers in the body.
This capability offers the opportunity to take samples from disease cohort repositories worldwide, where blood is stored from patients with a wide variety of diseases.
The derived iPSCs can serve as human cellular models for disease research and drug discovery. “The results suggest that iPSC lines can be generated from stored disease cohorts to make models to study a variety of diseases,” says Parker.
By generating iPSCs from people with diverse ethnic and genetic backgrounds, or from those who react poorly to certain drugs, scientists can better understand how drug compounds work. The next step is personalized medicine. “We can create models to study individuals to learn more about their disease and how best to treat them,” says Parker.
CDI’s overall business model—to create iPSC lines and manufacture them in industrial quantity, quality, and purity—represents an open system approach that the company strives to make widely available.
“When you look at the stem cell space, that’s a unique approach. When pharma customers spend time with us, they realize that we can put a powerful model system in their hands that works for them,” says Palay.

Immunostaining of iCell Neurons for the neuronal marker ß-111 tubulin (green) showing extensive neurite