
Fear of deadly contagion drives the action in The Hot Zone, a television drama inspired by the true story of Ebola’s first arrival in the United States in 1989. Although the Ebola strain that breached our borders back then was eventually found to be incapable of causing illness in humans, The Hot Zone recreates flashback scenes depicting the terrifying 1976 Ebola outbreak in Africa before cutting back to the spectacle of hazmat-suited U.S. Army personnel euthanizing infected animals and decontaminating lab facilities.
More recent African outbreaks attest to the deadly nature of this hemorrhagic fever and other emerging infectious diseases and highlight the diligent efforts to create new therapies. As in The Hot Zone, the challenges faced by everyday scientists sometimes prompt them to rise to the occasion and become real-life microbe-fighting heroes. They are already making headway developing novel immunotherapeutic strategies to attack and neutralize menacing and highly infectious viruses. (Immunotherapy is also targeting other areas from cancer to antibiotic resistance.)
The inaugural “Immunotherapy for Infectious Diseases” conference was recently held “to provide a forum for exchange of results and ideas and to establish a community of interested parties in the interphase between academia and industry,” commented meeting organizer Magnus Hook, PhD, professor, director, Center for Infectious and Inflammatory Diseases, Texas A&M Health Center. A second conference is planned for 2020 in Italy.
Conference topics included coaxing lung cells to combat infections, leveraging new technological innovations to create mRNA vaccines, assessing unique therapeutic monoclonal antibodies (mAbs) against Ebola, Zika, and other infectious diseases, and utilizing electroporation-assisted delivery of naked DNA to create “designer DNA vaccines.”
Luring lungs to attack pathogens
The epithelium of the lung, akin to a military perimeter, defends against infection both as a passive barrier and as a battery of active killing components, noted Burton F. Dickey, MD, professor and chair of the department of pulmonary medicine at the University of Texas MD Anderson Cancer Center. “Rather than outsourcing all active antimicrobial defenses to leukocytes, barrier epithelial cells, which face the onslaught of pathogen attack, have developed their own potent innate defenses,” Dickey explained.
Dickey along with Scott E. Evans, MD, associate professor in the same department, and colleagues, developed a strategy to induce lung cells to ramp up their defenses. The team identified a synergistic combination of two Toll-like receptor (TLR) agonists that, when inhaled, could induce rapid lung resistance to infection from more than 15 bacteria, viruses, and fungi.
According to Dickey, the discovery was exciting, but also puzzling. “There was,” Dickey pointed out, “no obvious link between the two agonists that consisted of a diacylated lipopeptide ligand for TLR2/6 (that is, Pam2CSK4) and a class C unmethylated 2′-deoxyribocytidine-phosphate-guanosine (CpG) ligand for TLR9 (that is, ODN M362).
The investigators next pursued the biology of the effect. “The combo (Pam2-ODN) induced production of reactive oxygen species without reliance on type I interferon signaling. Essentially, the lung epithelial cells were producing ‘Clorox’ to kill pathogens,” quipped Dickey.
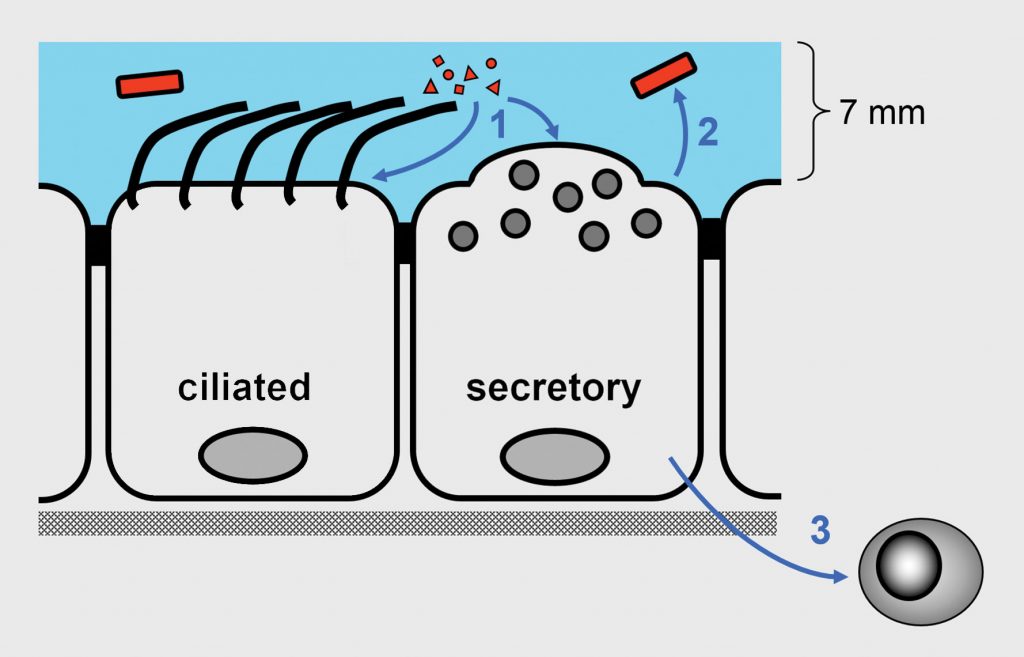
The scientists also recently discovered the mechanism of action, which presented another surprise. Dickey reported, “The ODN in the mix binds a cytoplasmic DNA sensor that is required for the rather magical effect in which all three players are engaged to induce pathogen killing.”
The PAM2-ODN therapeutic is currently in a Phase IIa trial. “We are testing its ability to block bronchitis caused by rhinovirus in a challenge study,” Dickey said. However, other uses include treating cancer patients undergoing myeloablative chemotherapy, who are very susceptible to pneumonia, as well as organ transplantation patients and others on immunosuppressants. Dickey concluded that “there are many strategies that could be developed, now that we know combinations of innate ligands delivered by aerosol to the lungs are capable of inducing a high level of broad host resistance against a variety of pathogens.”
mRNA vaccines: The body as bioreactor
Billions of people worldwide are at risk from endemic and newly emerging tropical infectious diseases. Although traditional vaccines have had an enormous impact on preventing disease and saving lives, hurdles remain to more rapid vaccine development and deployment.
Some believe that the introduction of mRNA vaccines could usher in a new era in vaccinology. Although early reports of successful use of in vitro transcribed mRNA in animals appeared more than 30 years ago, only recently have major technological innovations allowed mRNA to begin taking its place as a viable therapeutic.
“Typical vaccines often utilize recombinant proteins, but the need to produce and purify them are major hurdles,” explained Jeroen Pollet, PhD, assistant professor of pediatrics at the National School of Tropical Medicine at Baylor College of Medicine in Houston. “Once a platform is developed, the process can be streamlined. It is even conceivable to combine mRNAs against different antigens to increase potency.”
According to Pollet, mRNA vaccines offer some significant advantages: “There is no risk of genomic integration. The cellular immune response can be regulated both by nucleoside modifications and delivery methods, and mRNA vaccines can be produced by rapid, inexpensive, and scalable means.”
Pollet and colleagues at Texas Children’s Hospital Center for Vaccine Development are studying Chagas disease, which is caused by the protozoan parasite Trypanosoma cruzi. An estimated eight million people in Latin America are afflicted with the parasite, and the incidence is increasing. Pollet explained his approach: “We created a vaccine that combined six unique mRNAs encoding different parasite proteins and administered that to mice. We’ve had exciting results thus far; however, our in vivo experiments are complex because we aim to affect the lengthy chronic phase of Chagas disease.”
Pollet reported that this same strategy is being adapted to develop therapeutics for other infectious diseases such as Zika and rabies. In addition, Pollet pointed out that there are other lucrative uses of the technology: “Any therapeutic mAb could be developed into an mRNA-encoded antibody therapy. This would allow patients to make their own Abs in any of their transfected cells. Certainly, progress in overcoming challenges related to mRNA stability, immunogenicity, and delivery can now begin to drive a large and expanding commercial application of mRNA therapeutics.”
Consortium for standardizing mAbs
Although a number of investigators have produced and evaluated mAbs against Ebola virus (EBOV), making comparisons is challenging without standardization of assays and interpretations among the various groups. To help solve the problem, the Viral Hemorrhagic Fever Immunotherapeutic Consortium (VIC) assembled with the goal of gathering a broad pool of antibodies to EBOV and other viruses and analyzing them using a systematic strategy employing identical assay conditions. Gary P. Kobinger, PhD, professor and director of the Infectious Disease Research Centre at the Université Laval is a member of VIC. He gave the keynote address at the conference titled, “The Ascent of mAb Therapies against Infectious Diseases.”
Issues confronting mAb therapeutics include identifying which in vitro tests best predict the in vivo efficacy of mAbs. VIC recently published a report describing development of a comprehensive dataset examining more than 170 mAbs evaluated in each of 30 assays. The various mAbs included chimeric Abs, human survivor mAbs, and those raised by immunization. They concluded that no single neutralization assay alone can always predict protection, and that the mAb epitope is not the sole determinant of neutralization behavior. Despite these findings, the group compiled other sets of key information to serve as a framework for future studies of EBOV and other human pathogens.
Single human mAb quells Ebola
At the National Institute of Allergy and Infectious Diseases (NIAID) Vaccine Research Center (VRC), Nancy J. Sullivan, PhD, chief of the Biodefense Research Section, and colleagues have developed a therapeutic mAb (mAb114) derived from blood drawn from an Ebola survivor 11 years after infection.
Sullivan and team first verified the presence of circulating antibodies against the EBOV’s surface glycoprotein (GP). Then, they sorted the patient’s memory B cells, immortalized individual clones, and chose one with specific properties they had determined from previous research to increase mAb potency. After cloning its gene, purifying mAb protein, and testing in a rhesus macaque model, they found mAb114 could protect against infection even when given five days after challenge.
Seeking to identify the structural and molecular basis for mAb114’s striking activity, Sullivan and colleagues at Dartmouth College obtained crystals and solved the ternary structure of the mAb/GP complex. Sullivan explained that the GP, found in abundance on the virus surface, consists of a trimer of monomers with two subunits, GP1 and GP2. They found the mAb binds to a novel site of vulnerability on GP1, attesting to the value of identifying natural defenses targeted by the host immune system.
Subsequently, Julie E. Ledgerwood, DO, chief, clinical trials program at the VRC, and her team, led by Martin Gaudinski, MD, medical director at the VRC, completed the first-in-human open label-Phase I trial of mAb114. Ledgerwood reported (Lancet 2019) that even after a single 30-minute intravenous infusion (and patient monitoring for 24 weeks), the mAb was well tolerated, showed linear pharmacokinetics, and was rapidly infused, making it suitable for rapid deployment as a treatment for outbreaks. Thus, one of the striking features of mAb114 is that a single infusion, rather than several over multiple days, protects the subject.
Ridgeback Biotherapeutics is leading further clinical development of mAb114. Sullivan also reported that due to a recent African outbreak of EBOV, Jean Jacques Muyembe, director general of the Institut National de Recherche Biomédicale in Kinshasa, DRC, is treating patients under a WHO compassionate use protocol.
Designer DNA immunotherapies
Another form of immunotherapy, DNA vaccines, sparked interest in the scientific community in the 1990s with the technology’s theoretical ability to generate broad immune responses without the need for live attenuated virus. However, early clinical trials were disappointing, despite a good safety record. Recently, many of those hurdles have been overcome, propelling “designer DNA vaccines” once again into the limelight.
“Some of the earlier challenges were low response rates in humans, poor reproducibility, and lack of an immunogenic response,” reported David B. Weiner, PhD, executive vice president, director of the Vaccine & Immunotherapy Center, and W.W. Smith Charitable Trust Professor in Cancer Research at the Wistar Institute. Weiner, a pioneer in the field, said several key innovations are driving the new resurgence of interest: “First, we can now engineer inserts into DNA plasmids that will result in 25–50 times more protein expression per cell. Second, we’ve learned to increase DNA formulations that also have reduced volume. Third, we’ve dramatically increased our delivery with advanced electroporation-assisted devices that provide for cellular update of plasmid more than 1000-fold as compared to plasmid delivery alone.”
A search on clinicaltrials.gov revealed 610 current clinical trials using DNA vaccines for the treatment of cancers, influenza, and infectious diseases. Weiner feels that DNA vaccines provide a valuable immunotherapy especially for rapidly emerging infectious diseases. He gave the example of Zika virus. “During the 2015 outbreak, there were no drugs and no vaccine available to treat Zika,” he recalled. “Our vaccine, a synthetic DNA cassette featuring a Zika-specific antigen, was the first into the clinic and took only about 6.5 months to develop from bench to bedside.”
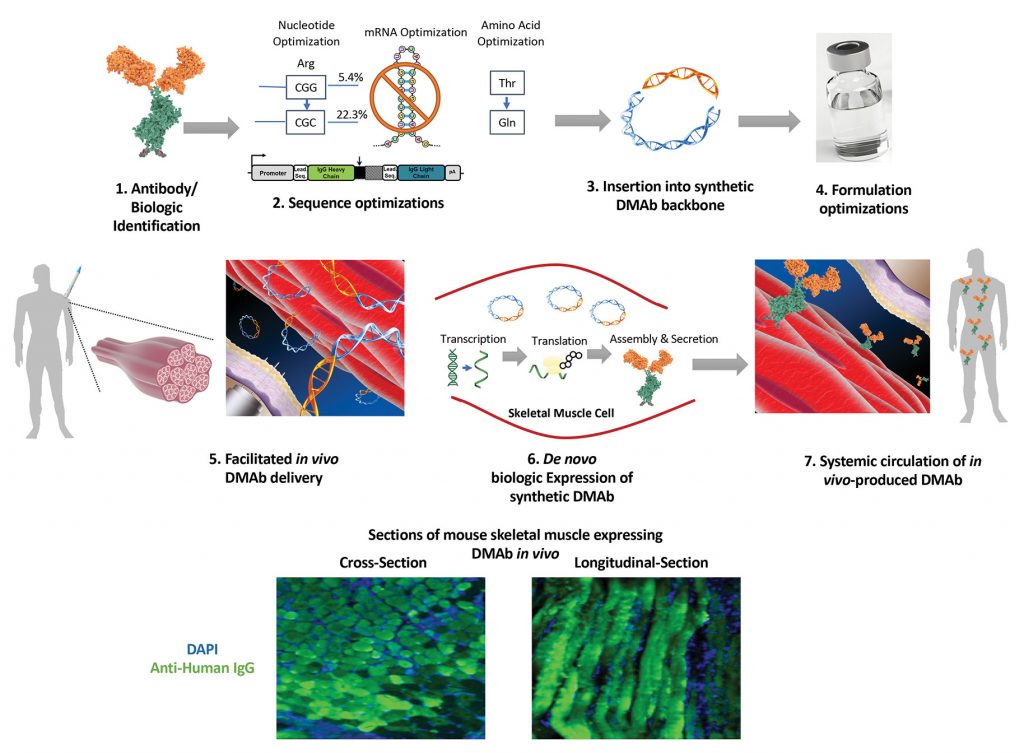
However, Weiner and colleagues have also taken another approach to fighting Zika. They have engineered designer synthetic plasmids with a DNA-encoding mAb, ZK190, that can produce a full-length functional antibody known to potently neutralize Zika in animal studies. The team found that when delivered in vivo, the DMAb-ZK190 was produced in the living animal and proved protective to Zika challenge in both mice and rhesus macaques. Weiner explained, “Unlike viral vector platforms, this platform is nonlive, nonintegrating, and noninfectious while promoting rapid and transient highly targeted DMAb generation.”


Comments are closed.