November 15, 2015 (Vol. 35, No. 20)
New Research Tools Assess Overall Cell Health
Careful selection of fluorescent products can allow for one simple experiment that can identify the healthy, dying, and dead cell populations, in addition to determining which portion of that cell population is undergoing apoptosis versus necrosis. In ImmunoChemistry Technologies’ (ICT) Necrosis vs. Apoptosis Assay Kit, detection of cell membrane integrity loss, indicative of necrosis or late-stage apoptosis, is facilitated by the addition of the vital staining dye, 7-aminoactinomycin D (7-AAD), a red fluorescing live/dead stain.
This vital dye works by penetrating cell membrane-compromised cells and tightly binding to GC rich regions of the DNA. Because 7-AAD alone cannot detect cells in the early stages of apoptosis, the Necrosis vs Apoptosis Assay Kit combines 7-AAD with a green-fluorescent FLICA apoptosis detection reagent. Combining these two different types of fluorescent cell-status-indicator reagents within a single testing format enables the specific detection of healthy cells (FLICA− and 7-AAD−), cells in early-stage apoptosis (FLICA+ and 7-AAD−), cells in late-stage apoptosis (FLICA+ and 7-AAD+), and necrotic cells (FLICA− and 7-AAD+). This differential staining pattern is illustrated in Figures 1 and 2.
Improper handling and maintenance of cell culture concentrations can lead to unhealthy cells that have the potential to introduce artifacts or difficult to interpret results. It’s best to avoid situations where cell suspension populations fall below 5×104 cells/mL or adherent cell confluency levels fall below 20%. Another common mistake is to allow cells to become overgrown prior to use in experimentation. Such a situation can give rise to an increased number of cells that have naturally initiated the process of apoptosis.
Over time, improper culturing practices can also select for cells that are more fit to handle harsh environmental conditions, leading to a genetic subset that may or may not respond to the experimental treatment regimen as would the general clonal population. We generally recommend that suspension cell cultures designated for use as live cell indicators for monitoring the effects of a particular experimental treatment never be grown at cell density levels exceeding 1×106 cells/mL for extended periods of time.
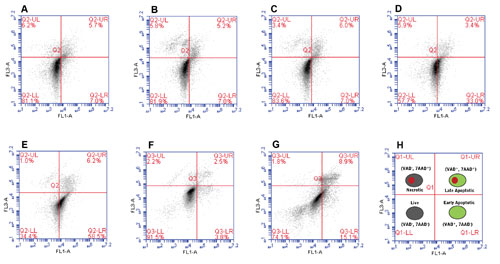
Figure 1 demonstrates the effect overgrowth has on the portion of the cell population that is apoptotic. Suspension cells maintained at a concentration between 1×105 and 6×105 cells/mL appeared healthy, with > 80% of the population staining as FLICA− and 7-AAD−. Cell cultures allowed to reach a concentration of 1×106 cells/mL showed considerably more cells having entered into early apoptosis (FLICA+ and 7-AAD−) compared to cells maintained at a 10-fold lower concentration of 1×105 cells/mL (33.0% versus 7.0%, respectively).
The percent of cells that were in early or late apoptosis continued to increase as the cell concentration surpassed 1×106 cells/mL (Figure 1E). Similarly, adherent epithelial cells grown to 100% confluency showed higher numbers of cells entering early-stage and late-stage apoptosis compared to cells grown to 80% confluency (Figure 1F–1G).
Suspension or adherent cell cultures designated to be used as indicators of the experimental treatment, must be routinely cultured in an optimal cell culture media. Suspension cell concentrations or adherent cell confluency levels must be regulated to avoid the deleterious effects that overcrowding can cause. Inattentive monitoring of cell density or confluency levels represents the most common source of unintended cellular stress exposure.

Figure 1. Effect of cell density on the percentage of healthy, early-stage apoptotic, late-stage apoptotic, and necrotic cells. Jurkat cells were grown to (A) 1×105, (B) 4×105, (C) 6×105, (D) 1 x106, and (E) 3×106 cells/mL prior to staining with the Necrosis vs. Apoptosis Assay Kit and analysis by flow cytometry. The adherent cell line, U-2 OS, was grown until (F) less than 80% confluent or (G) approximately 100% confluent prior to staining with the Necrosis vs. Apoptosis Assay Kit and analysis by flow cytometry. The key is shown in (H).
Undesirable events arising from routine cultivation of the treatment indicator cells in suboptimal cell culture media or allowing cell density conditions to fall outside the optimal concentration density range include: 1) selection of a particular genetic clonal subset within the genotypically identical cell population, 2) loss or reduction of individual cell(s) ability to respond normally to a particular experimental treatment, 3) acceleration of spontaneous apoptosis induction rates within the cell population, and 4) excessive levels of necrotic cells, most of which arise from end-stage apoptosis.
Adoption of ICT’s newly restructured apoptosis/necrosis format as an in-house quality control protocol for monitoring the ongoing health status of your treatment-indicator cells, can help you determine in advance whether or not your indicator cells are capable of providing you with accurate results.
The FLICA probe component of the Necrosis vs. Apoptosis Assay kit can be used to assess the baseline percentage of spontaneous apoptosis. For example, spontaneous induction percentages exceeding 20% could serve as a red flag to avoid using such a cell population for experimentation.
Likewise, the 7-AAD component can detect baseline levels of necrosis or end-stage apoptosis in a readout indicator cell population. Depending on the particular cell line, a baseline percentage of late-stage apoptosis (FLICA+ and 7-AAD+) or end-stage apoptosis or necrosis (FLICA− and 7-AAD+) greater than 20% would suggest that this readout indicator cell population was unfit for experimental data generation. Tools such as this are useful not only for monitoring overall cell health, but also for assessing cytotoxic response to biological or chemical treatments.
Given the time and expense that goes into all cell culture-based research, it is wise to make every effort on the front end of project development to assure that the cell lines used to monitor experimental results are in a good physiological state. The new ImmunoChemistry Technologies Necrosis vs. Apoptosis Assay Kit can assist researchers in this endeavor.

Figure 2. Fluorescence microscopy imaging of apoptotic cells stained with the Necrosis vs. Apoptosis Assay Kit. Jurkat cells were grown to 5×105 cells/mL and then induced to become apoptotic by exposure to 1 µM staurosporine for 4 hours at 37°C. (A) Early-stage apoptotic cells exhibit a cell-retained green fluorescence following exposure to both the green FLICA and red 7-AAD. Cell-membrane-compromised necrotic and very late-stage apoptotic cells exhibit red (7-AAD-only) fluorescence. Green and red fluorescing cells represent the population of Jurkat cells in mid- to late-stage apoptosis. (B) A corresponding differential interference contrast (DIC) image was included to show cell morphology. Images were obtained using an Olympus BH-2 photomicroscope equipped with bright field, DIC, and fluorescence optics. Both the carboxyfluorescein (green dye) and 7-AAD (red dye) labels were imaged using a 470–490 nm excitation filter plus >520 nm long pass filter tandem.
Kristi Strandberg, Ph.D., is staff scientist, Tracy Murphy is director of research and production, and Brian Lee, Ph.D. ([email protected]), is president at ImmunoChemistry Technologies.