On the 20th anniversary of the publications heralding the first drafts of the human genome sequence, GEN revisits the bold 40-year outlook then offered by Francis Collins.

Not everyone is Nostradamus. However, some people’s predictions have turned out to be eerily accurate. For example, in his 1968 novel 2001: A Space Odyssey, Arthur C. Clarke predicted the use of the “newspad,” an object very similar to today’s iPad, which was released in 2010. Similarly, in 1909, Nikola Tesla said, “It will soon be possible to transmit wireless messages all over the world so simply that any individual can carry and operate his own apparatus.” And, when Alexander Graham Bell wasn’t inventing the telephone, he was forecasting climate change. In 1917, he wrote about the “sort of greenhouse effect” that burning fossil fuels would have. But not everyone has 20:20 vision. In 1981, Bill Gates famously goofed when he said, “640K ought to be enough for anybody.”
Making predictions about the future of science may seem like a fool’s errand. But 20 years ago, when the first drafts of the human genome were published, many scientists were inclined to prophesy, regardless of the risks. It was a heady time. One draft was published in Nature by the International Human Genome Sequencing Consortium. A rival draft was published in Science by Celera Genomics.
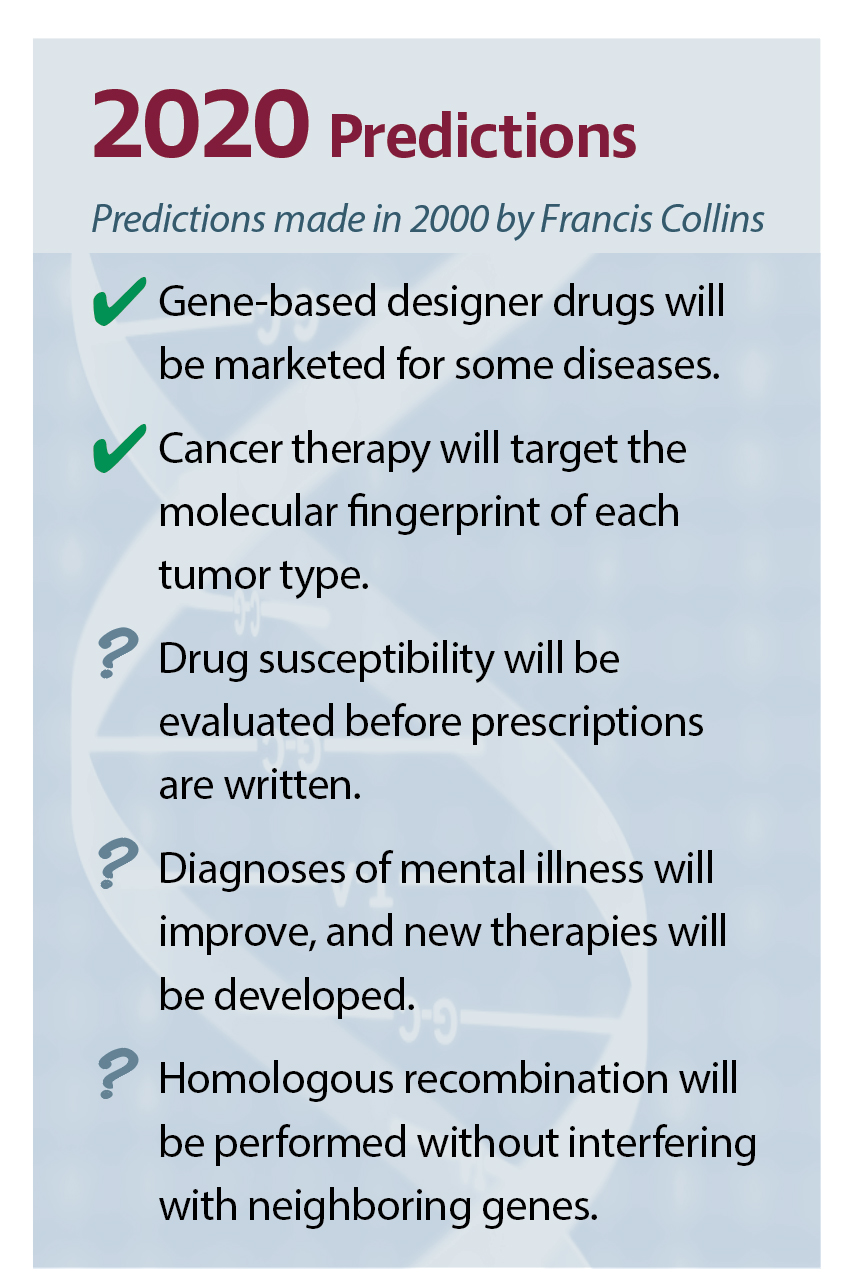
 At the time, Francis Collins, MD, PhD, the current director of the National Institutes of Health (NIH), was at the helm of the National Human Genome Research Institute (NHGRI), a leading participant in the international consortium. Around the time that the draft sequences appeared, Collins predicted the milestones that genomics would pass, decade by decade, all the way to 2040 (see sidebars).
At the time, Francis Collins, MD, PhD, the current director of the National Institutes of Health (NIH), was at the helm of the National Human Genome Research Institute (NHGRI), a leading participant in the international consortium. Around the time that the draft sequences appeared, Collins predicted the milestones that genomics would pass, decade by decade, all the way to 2040 (see sidebars).
Offering a glimpse into Collins’ motivation, Eric Green, MD, PhD, the current director of the NHGRI, explains that when the Human Genome Project began, “Its goals were audacious and, many thought, unreachable.” In the end, those goals were achieved on a compressed timeline, sparking the realization that bold and daring objectives serve to catalyze innovation and progress. That legacy of attaining seemingly out-of-reach goals has prompted the subsequent use of bold predictions as a source of inspiration, a roadmap for future endeavors, and a tool for benchmarking accomplishments.
Twenty years later, we look back on some of those predictions by asking experts how close Collins’ predictions were, and what hurdles need to be cleared to bring them to fruition. Each section below highlights a different prediction.
In 2010…
the National Coalition for Health Professional Education in Genetics will promote genetic literacy
According to Laura Hercher, the director of research in human genetics at Sarah Lawrence College and host of “The Beagle Has Landed” podcast, Collins was prescient in suggesting that the National Coalition for Health Professional Education in Genetics (NCHPEG) would be an important aspect of the precision medicine initiative, to “promote genetic literacy for physicians and nurses.” Unfortunately, the NCHPEG closed due to funding shortages in 2013, but other initiatives have emerged for training medical students and practicing physicians.
 Have they been successful? Hercher answers, “In truth, we remain well short of the goal.” Doctors continue to report that they are not comfortable interpreting or contextualizing genetic information. Although genetic testing has become routine across a growing range of specialties, variant interpretation is not a standard part of medical school curricula.
Have they been successful? Hercher answers, “In truth, we remain well short of the goal.” Doctors continue to report that they are not comfortable interpreting or contextualizing genetic information. Although genetic testing has become routine across a growing range of specialties, variant interpretation is not a standard part of medical school curricula.
Most alarmingly, we see evidence that the results of genetic testing are being misinterpreted even in areas where it has become standard of practice. In oncology, for example, where breast cancer susceptibility testing has been done for decades, a recent study showed that a significant number of patients with a BRCA1 or BRCA2 variant of uncertain significance (VUS) were undergoing bilateral mastectomy, an indication that their surgeons overestimated the risk associated with a VUS.
Hercher tells GEN that in the United States today, there are fewer than 5,000 genetic counselors and one-quarter that number of medical geneticists. She asserts that ambitious plans for the expansion of genomic medicine into routine care will meet a hard cap if they rely on genetic professionals to identify those at risk or explain the results of testing.
In 2020…
cancer therapy will target the molecular fingerprint of each tumor type
Collins’ optimistic take on the role that genetics would play in cancer treatment prompted us to seek expert commentary from Jesse Salk, MD, PhD, co-founder and CEO at TwinStrand Biosciences. The company recently introduced Duplex Sequencing, a technology that provides highly accurate reads and makes it possible to detect low-frequency variants—even one from a single cancer cell among 100,000 normal cells.

TwinStrand Biosciences
Salk tells GEN that Collins’ prediction “was actually more on the mark than he probably realized at the time.” He notes that over the past five years, multiple different types of “fingerprints” have been discovered. “These reflect a diversity of processes that lead to a tumor’s formation,” he adds. “[They] are increasingly relevant for predicting the cancer’s behavior and response to treatment.”
As a practicing oncologist at the Veterans Affairs hospital affiliated with the University of Washington, Salk suspects that the initial prediction envisioned identifying the particular genes mutated (or transcriptional patterns disrupted) in each tumor and directing treatments at inhibiting specific growth pathways. “We routinely use many such molecular markers in clinical decision making to pick targeted therapies,” he remarks.
 A few examples among many include RAS pathway mutations in colorectal cancer, EGFR pathway mutations in lung cancer, and HER2 amplification in breast and esophageal cancer. “In breast cancer,” he continues, “we commonly use expression patterns to risk-stratify localized tumors to inform decisions about adjuvant cytotoxic therapy.”
A few examples among many include RAS pathway mutations in colorectal cancer, EGFR pathway mutations in lung cancer, and HER2 amplification in breast and esophageal cancer. “In breast cancer,” he continues, “we commonly use expression patterns to risk-stratify localized tumors to inform decisions about adjuvant cytotoxic therapy.”
However, the notion of “fingerprints” now extends far deeper. Many of the mechanisms that help certain tumors hypermutate and evolve quickly leave telltale signs in the genome and can be exploited when it comes to synthetic lethality of DNA-damaging agents or immunotherapies. For example, he explains, “if a high tumor mutation burden fingerprint were found, I would use checkpoint inhibitors as a treatment. In contrast, a BRCA-like double-strand break repair–deficient fingerprint would warrant the application of platinum agents or PARP inhibitors and screening for germline inheritance.”
Even more complex types of mutational fingerprints are currently being investigated. In recent years, significant efforts have been put into investigating the underlying mutagenic processes that contribute to malignancy. Building upon that knowledge, TwinStrand is currently researching these signatures in people without cancer to determine if they have been exposed to environmental mutagens that could put them at risk for cancer.
Although mutation information is used to inform treatment planning, and could be used to inform risk determination, Collins’ vision has not been fully realized. It is likely, according to Salk, that only a minority of genetic fingerprints have been discovered, and that more remain to be found in the epigenome, transcriptome, proteome, and glycome. Salk reckons that even when more fingerprints are characterized, oncology will still be limited by the number of available treatments: “Phenomenological discovery typically predates our ability to intervene, but new therapies are predicated on discovery. So, continuing research on both is essential.”
In 2020…
drug susceptibility will be evaluated before prescriptions are written
According to Mary Relling, PharmD, chair of the Pharmaceutical Department at St. Jude Children’s Research Hospital, there are a few programs—including a comprehensive one at St. Jude’s—that have incorporated preemptive pharmacogenomic testing prior to prescriptions being written. It is apparent, then, that Collins’ prediction about drug susceptibility testing has been borne out, if only in part. “Such testing,” Relling notes, “remains relatively rare in the United States and elsewhere.”
 Testing preemptively with a multigene panel makes sense, Relling says, because (a) testing is relatively inexpensive, and the cost to test multiple genes is not much greater than a single gene; (b) most patients have at least one high-risk pharmacogenetic type; (c) most patients over their lifetime will be prescribed a high-risk drug; and (d) tests work best when the results are already in hand at the time of prescribing, without the “speed bump” of ordering and waiting for a test result.
Testing preemptively with a multigene panel makes sense, Relling says, because (a) testing is relatively inexpensive, and the cost to test multiple genes is not much greater than a single gene; (b) most patients have at least one high-risk pharmacogenetic type; (c) most patients over their lifetime will be prescribed a high-risk drug; and (d) tests work best when the results are already in hand at the time of prescribing, without the “speed bump” of ordering and waiting for a test result.
Some of the hurdles that have hampered testing include clinicians not knowing how to act on test results. Groups like the Clinical Pharmacogenetics Implementation Consortium have tried to address this need by providing freely available, evidence-based guidelines on how to use genetic tests to guide prescribing. Furthermore, pharmacogenetic tests are not always well reimbursed by payors; this hurdle has been partly addressed by favorable local coverage decisions for pharmacogenetic testing.
In 2030…
complete genome sequencing will cost less than $1,000/person

Element Biosciences
Genome sequencing technology has moved more quickly than most would have guessed—including Collins. He predicted that the $1,000 genome would be reached by 2030. But Illumina claimed to have reached that threshold in 2014. Today, we can easily anticipate reaching a $10 human genome by 2030.
The downward trend’s history is familiar to Molly He, PhD, co-founder and CEO of Element Biosciences, because she once worked at both Pacific Biosciences and Illumina. “The cost per data point has gone down so quickly thanks to Illumina’s success,” she tells GEN. “Illumina’s highly parallelizable sequencing technology enabled orders of magnitude more data in the same run. In addition, innovations in material sciences, enzymology, and chemistry have allowed significant cost reduction in reagents.”
 Last year, at the Advances in Genome Biology and Technology (AGBT) meeting held in Marco Island, FL, MGI presented the Tx—a new DNBseq instrument with new antibody-based chemistry. These advances, the company noted, allowed the production of the first $100 genome. In a recent preprint posted on bioRxiv, MGI described another competitive solution, one that yields a $15 genome using tape-based technology and robotic factory.
Last year, at the Advances in Genome Biology and Technology (AGBT) meeting held in Marco Island, FL, MGI presented the Tx—a new DNBseq instrument with new antibody-based chemistry. These advances, the company noted, allowed the production of the first $100 genome. In a recent preprint posted on bioRxiv, MGI described another competitive solution, one that yields a $15 genome using tape-based technology and robotic factory.
“[This] robotic factory is an interesting engineering twist to drive price down by increasing throughput,” she notes. Another way to reduce costs, she suggests, is to use a completely different technology that would fundamentally change how sequencing is done at the component level. It is possible to accomplish this daunting task, but it would require disruptive changes to the fundamental elements of the platform from surface chemistry to detection systems.

In 2030…
human aging genes will be fully catalogued, and clinical trials to extend human lifespan will be ongoing
Aging expert Leonard Guarente, PhD, tells GEN, “I think Dr. Collins’ 2000 prediction was a safe one and is on track to be realized.” Guarente is director at the Paul F. Glenn Center for Biology of Aging Research at MIT, and co-founder and chief scientist of Elysium Health.

MIT/Elysium Health
The Elysium website says that the company focuses on “exploring compelling scientific advancements that have the potential to benefit everyone.” Elysium sells supplements, one of which is a B vitamin complex including omega-3 fatty acids and antioxidants. The company asserts that this supplement slows age-related grey matter atrophy.
Guarente continues, “We have identified a handful of genes that have been shown to broadly influence aging and lifespan in diverse model organisms, including mice. The list of additional genes that influence more specific aspects of aging will continue to grow over the next decade.”
We have also witnessed, he continues, the emergence of useful biomarkers that assess the rate of aging in people, most notably DNA methylation–based clocks. Guarente expects that in the next several years, he will see “human data based on interventions that affect these aging genes, for example, NAD+ boosters to activate sirtuins, combined with aging clocks and physiological measurements that determine whether human aging can be slowed down or even reversed.”
In 2040…
gene therapies and gene-based drug therapies will be available for most diseases
Collins made two predictions about gene therapy. The first was that by 2010, gene therapy would be successful for a few conditions. This prediction was realized just a little later than he expected. To date, the FDA has approved two gene therapies. In 2017, Luxturna was approved for a rare inherited blindness; in 2019, Zolgensma was approved for children with spinal muscular atrophy.

AskBio
Currently, many hundreds of therapies are in the pipeline, signaling that gene therapy is turning a corner. No one knows that better than Sheila Mikhail, JD, CEO of AskBio, the gene therapy company recently acquired by Bayer for $2 billion. GEN asked Mikhail for her perspective on Collins’ second prediction about gene therapy—that in 2040, gene therapies and gene-based drug therapies will be available for most diseases.
“The vision for a world without genetic disease feels within reach,” states Mikhail. She adds that discoveries and collaborative research in gene therapy are changing the face of healthcare, and that the prospects for patients are evolving incredibly fast. Building upon these foundational discoveries to advance adeno-associated virus (AAV) technology in particular will redefine what the treatment of genetic diseases should be, and bring us closer to the ultimate goal of erasing them in our lifetime.
 Will it take another 20 years, given Collins’ timeline? Mikhail answers, “Just look at how far the industry has come in the last 20 years.” Not so long ago, she observes, gene therapy was considered science fiction, with practically no research funding. But gene therapy is now one of the most promising and compelling areas in the biotech space, Mikhail continues, one that generates massive investment.
Will it take another 20 years, given Collins’ timeline? Mikhail answers, “Just look at how far the industry has come in the last 20 years.” Not so long ago, she observes, gene therapy was considered science fiction, with practically no research funding. But gene therapy is now one of the most promising and compelling areas in the biotech space, Mikhail continues, one that generates massive investment.
Innovation in capsid reengineering and promoter design, coupled with scaled manufacturing processes, means there are new tools to provide genetic therapies to more patients suffering from a wider spectrum of diseases. There’s a significant opportunity to help patients whose medical needs are not yet met by today’s treatment options.
One remaining hurdle, Mikhail points out, is the need to deal with the immune system’s response to AAV vectors. This response can interfere with long-term efficacy and safety, especially if high vector doses are delivered to the liver. In the past 20 years, Mikhail notes, the science for finding missing or mutated genes to correct genetic disorders has greatly evolved, making genetic medicine more accurate, safe, and effective than it was decades ago.
The point of predicting
In a fast-moving field like genomics, notes Green, developing new strategic visions are important for taking stock of current scientific capabilities and for galvanizing excitement about new opportunities. Looking back at previous goals and predictions is helpful, he adds, “as a reminder of recent accomplishments and as a tool for identifying the most compelling research paths going forward.” Green asserts that such predictions also act as a reminder to the genomics community, the broader research community, and society as a whole that there remain many genomic research challenges left to be conquered.
Now that we are at the halfway point through Collins’ predictions, it appears that they have been fairly accurate overall. Although they cover a lot of topics, they are silent with respect to genome editing’s impact as a research tool. This omission is ironic, given that Collins’ recent work includes a significant research advance: the use of base editing to successfully treat progeria—a form of premature aging—in a mouse model.
How will his predictions for the next two decades hold up as the field of genomics hurtles ahead? Time will tell. If that answer seems unsatisfactory, remember what Albert Einstein said: “I never think of the future. It comes soon enough.” Regardless of whether Collins’ predictions hit all their marks, thinking critically about the role of genomics in our society is a worthwhile—and fun—exercise.




