
CEO, Impacts.Ca
As the microbiome therapeutics market continues to draw interest from both industry and consumers, it is evolving in new directions. Four major trends in the market have been identified, and they will be explored in this article. These include: 1) an expansion of microbiome therapeutics beyond the digestive system, 2) an increase in consumer awareness, 3) a rise in interest from Big Pharma, and 4) an intensification of basic research and clinical development.
This article will also highlight data from an online survey that was coordinated late last year by Impacts.Ca. In October 2020, 600 individuals were surveyed on the topic of Microbiome Therapeutics, of which 60% were from the United States and 40% were from Europe. About two-thirds of the people surveyed said that they use microbiome products, reinforcing our sense that microbiome products are going mainstream.
Going beyond the digestive system
Traditionally, microbiome therapeutic products have been associated with the digestive system. Nonetheless, in the last two years, the first microbiome therapeutic trend—the growing importance of products that are relevant beyond the digestive system—has become increasingly evident.
There have been several collaborations in fields beyond the digestive system. Manufacturers and retailers have reported a surge in demand for immunity-boosting products (likely connected to the COVID-19 pandemic). There have also been increases in the use of probiotic supplements for vaginal health, in the use of dietary supplements for lifestyle conditions, and in the creation of partnerships to research the skin microbiome. For example, L’Oréal partnered with uBiome, and Evolve Biosystems partnered with Janssen Research & Development.
According to survey participants, the digestive system is still central to microbiome therapeutics; 75% think of the microbiome in terms of digestive and gut health. Nonetheless, several secondary markets have emerged; 50% of participants expressed interest in the benefits related to immunity, 25% mentioned weight loss, and 16% talked about hair growth and articulation pain. When participants were asked about everyday usage, they said that they purchased products mostly for digestive purposes, with a small cluster of participants purchasing products for skincare.
Consumer awareness
The second trend, the growth in consumer awareness, is noticeable in the ways that consumers are using the internet to find information about microbial therapeutics. A few years ago, consumers would enter generic search terms into internet search engines, terms such as “gut flora” or “digestive health.” Today, consumers are being more specific. They are even searching for specific bacterial strains. New web searches also focus on immunity, stress, anxiety, and the gut–brain axis. In fact, these outpace digestive system queries.
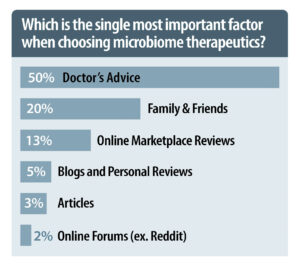
Information about consumers’ purchasing patterns from the literature and that from the survey diverge. Recent articles suggest that consumers’ selection processes are centered around reviews and consumer ratings. The survey, however, suggests that personal recommendations are a lot more important. Most participants indicated that they rely on the advice they receive. In fact, personal recommendations—from doctors, friends, and family members—were the single most important factor when choosing microbiome therapeutic products, accounting for 76% of answers, whereas the other sources such as online reviews and blog entries accounted for very few answers.
It was also interesting to compare what the literature and the survey had to say about the locations where products are most likely to be purchased. According to the literature, supermarkets and pharmacies are the main locations. In the survey, online marketplaces and online stores for probiotic markets accounted for 30% of answers, more than what the literature suggested. Survey participants also indicated that for them, physical retail stores and pharmacy drugstores are much less important.
Is this a post COVID-19 impact? It is reasonable to assume that the pandemic has shifted shopping patterns, contributing to the general trend towards online retailing. Products are purchased online a lot more often than they were one year ago.
Big Pharma and microbiome therapeutics
The third trend relates to the increasingly close relationship between Big Pharma and microbiome therapeutics. A few years ago, Big Pharma participated in a wave of partnering in this space. However, Big Pharma became more cautious after a few high-profile misses in clinical trials.
What we’ve been seeing lately, especially in the last two years, is a renewal of interest on the part of Big Pharma. The interest is evident mostly through collaborations, but there have been a few acquisitions, too. Big Pharma companies bring a lot to the table. They have experience in the regulatory space, product approval, and commercialization.
As such, there are many deals; however, most are backloaded. Typically, deals involve a small upfront payment, which may be succeeded by a huge payoff if a product reaches commercialization. Big companies such as DuPont, Boehringer, Takeda, Gilead, Novozymes, Pfizer, and Merck have all signed deals. We expect these types of deals to proliferate in the next few years.
Survey participants were also asked their opinion on Big Pharma’s presence in the microbiome therapeutic space. More than half of the participants were highly positive. About 18% of the participants expressed negative opinions about Big Pharma’s presence in the space. Some of these participants indicated that they believe pharmaceutical companies exploit health issues by overcharging for drugs.
The perception that Big Pharma seeks profiteering opportunities was evident in open-ended comments contributed to the survey. Accordingly, the negative sentiments detected in the survey may have less to do with the relationship between Big Pharma and microbiome therapeutics than they do with a general aversion some people have for Big Pharma. Overall, Big Pharma’s presence in this space is viewed positively by consumers who believe that the space needs more structure.
More clinical trials and research
The fourth microbiome therapeutics trend, namely, the intensification of basic research and clinical development, is evident in two basic metrics: the number of publications globally and the number of clinical trials in the United States. In 2010, there were 1,177 publications on the microbiome, which increased to 6,964 in 2015. In 2020, there were over 20,867 different publications around the microbiome therapeutic space.
Besides becoming more numerous, research efforts are attracting more funding and addressing a wider range of applications. For example, studies that could lead to microbiome therapeutics to treat obesity, autoimmune disease, and respiratory disease are becoming more common.
The increased pace of clinical development is evident in the growing number of clinical trials. In 2010, there were 31 studies open in the microbiome space. In 2015, that number increased to 271. In 2020, it reached 632.
Upcoming challenge: Individuality
Individuality is an important challenge in microbiome therapeutics. Because every individual’s microbiome has unique characteristics, microbiome studies face tricky questions: How do you define a healthy or unhealthy microbiome? How do you ensure that your clinical study includes participants that are representative of large patient populations, or subpopulations defined by factors such as age? How do changes in lifestyle—for example, changes in diet, physical activity, or medication use—affect clinical results?
 Despite the challenge of accommodating individuality, clinical studies will remain an important part of microbiome product development. It will also be an important part of commercialization. After all, clinical evidence is a key element consumers consider when they select products. The industry is moving toward a more mature, educated consumer base, and clinical evidence is playing a larger role than pricing and third-party reviews.
Despite the challenge of accommodating individuality, clinical studies will remain an important part of microbiome product development. It will also be an important part of commercialization. After all, clinical evidence is a key element consumers consider when they select products. The industry is moving toward a more mature, educated consumer base, and clinical evidence is playing a larger role than pricing and third-party reviews.
For example, the survey participants were asked if they were more likely than not to purchase a microbiome product that had completed clinical trials. And almost three-quarters of our participants were positive that clinical trials differentiate products in a purchasing decision.
Closing words
We’re looking at a maturing microbiome therapeutics market. We’re moving from single-indication products (digestive issues) to multiple-indication products (dysbioses involving the gut–brain axis, the skin microbiome, or the metabolic complications associated with obesity). We’re seeing that consumers are informing themselves and integrating what they learn into their purchasing decisions. We’re seeing that Big Pharma is expanding its presence in microbiome therapeutics, helping the field gain credibility. And we’re seeing a lot of moves from unregulated to regulated products. These trends suggest that microbiome therapeutic products are becoming more attractive, more mainstream.
Jean-François Denault ([email protected]) is the CEO of Montreal-based Impacts.Ca, a consulting firm specializing in emerging technologies, especially those impacting the life sciences.
