December 1, 2010 (Vol. 30, No. 21)
Scaleup, Process Performance, and Product Quality Serve as Key Points for Discussion
Scientists from industry and academia gathered in October to discuss biomanufacturing trends and technologies at “Cell Factories of the Future,” a symposium held at Rentschler Biotechnologie’s main facilities in Laupheim, Germany. The symposium was the first in a new series of specialized conferences.
Stefan Schlatter, Ph.D, associate director of upstream development at Boehringer Ingelheim (BI) presented a talk on BI-HEX® (Boehringer Ingelheim High Expression), a platform for optimizing CHO production of monoclonal antibodies “from DNA to commercial process.”
BI-HEX commits resources early in development to scaleup, process performance, and product quality. The focus is clone selection, but BI-HEX extends through commercial production, downstream processing, and formulation, leaning heavily on BI’s experience with CHO and mAb production.
“It’s an experience-based platform, a concept for the entire process consisting of preferred conditions and operations culled from our understanding of cell culture over the last several decades.”
BI-HEX reportedly results in specific productivity averaging more that 100 pg/cell/day, or final titers of about 8 g/L, for standard fed-batch cultures. The conditions are not widely publicized, but Dr. Schlatter said “the concept is no secret. The key is to achieve high productivity with high quality consistently and predictably, not just once and then selling it.”
BI-HEX supports BI’s own products but is also offered commercially, either as a full development service or up to the clone selection step. BI can also assume post-approval manufacturing, where it leans on its experience from the development stage.
Readers may wonder how sponsors, particularly development-stage companies, can afford to devote significant resources to preclinical molecules when most will fail anyway during clinical testing. That, said Dr. Schlatter, is a misrepresentation of what BI-HEX is about.
“Our slogan, ‘do it right the first time,’ can be misinterpreted. It doesn’t mean to cover every detail in the beginning, but to conduct development in such a way that steps need not be repeated or re-done later at larger scale. Do things in a way that they do not need to be redone later.”
With respect to cell-line development, arguably the critical step, Dr. Schlatter described the process as “commercially enabling.” He said BI takes great care during clone selection, paying extra attention to stable, highly productive, consistent and well-characterized cell lines with impurity profiles that facilitate purification.
“All three factors—scalability, productivity, and quality—factor into the decision of which clone is best.” These attributes are often balanced against each other, e.g., a high producer that does not scale, or a highly scalable cell line with high concentrations of a troublesome impurity. “The clone we select must be one that we can work with for the entire life cycle of the product.”
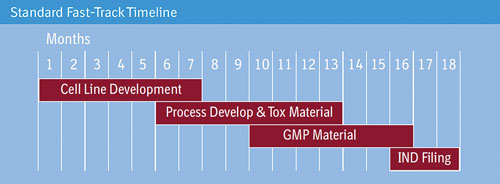
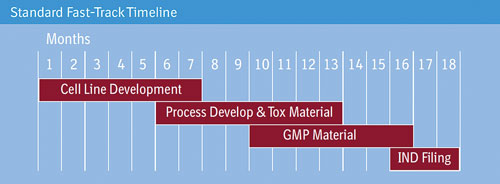
Boehringer Ingelheim says that its BI-HEX cell-culture process development platform allows processes to be fast-tracked.
Platform Technologies
Volker Sandig, Ph.D., CSO at ProBioGen, highlighted the importance of production platforms for manufacturing recombinant proteins, antibodies, and viruses. He noted that even under the best conditions, expressed proteins produced may interfere with host cell metabolism, be modified or degraded by the cell, or misfold or aggregate.
Dr. Sandig referred to these as “difficult proteins,” and described ways to deal with them by enhancing the folding apparatus, providing alternative cell substrates, or through selection and screening approaches.
Protein misfolding may occur despite intracellular mechanisms to prevent it, for example the glucosidase II system, the calnexin calreticulin system, chaperones, and enzymes such as peptididyl-prolyl-cis-trans isomerases and protein-disulfide isomerases. Misfolded proteins recognized by chaperones within the endoplasmatic reticulum may trigger the unfolded protein response—a crosstalk mechanism between the endoplasmic reticulum and nucleus, which affects proteins subsequently produced.
“Metabolic limitations of the specific producer cell can induce the unfolded response as well,” Dr. Sandig said, resulting in a shut-down of transgene transcription of difficult proteins, and limited expression of even well-expressed products. Molecular engineering can help cells overcome these challenges while maintaining expression.
What does selection mean in the context of dealing with difficult proteins? As an integral element generating high-producing clones, ProBiogen scientists use drugs that identify cells that carry the desired transgene and marker gene conferring resistance to the drug. High drug concentrations are more easily tolerated by cells that express high marker and transgene levels.
Enhancing the folding apparatus is another hallmark of this approach. Cells are equipped either for rapid proliferation or for production of secreted proteins. Changes occurring in a mature plasma cell are the most prominent examples of the latter. While introduction of individual chaperones has mostly failed to improve the yield of secreted proteins, the co-expression of specific modulators affecting secretion have shown benefit.
“We have evaluated a number of such candidate modulators identifying ones with prominent effects. However, the benefit of the specific regulator is closely linked to the individual starter cell and requires extensive fine-tuning,” explained Dr. Sandig.
Currently, CHO are the workhorse cells for manufacturing biologics. Expression of some proteins is compromised in CHO as a result of signal transduction or CHO-specific degradation or modification. In these instances other cell types may overcome this hurdle in much less time than it takes to optimize a CHO line.
“We repeatedly noted this benefit with designed human and duck cell lines of the AGE1 platform. Of course, alternative cell substrates require documentation for origin and history, and possess the usual characteristics of top-performing pharmaceutical producer cell lines, including suspension growth in chemically defined media.”
Art and Science
Despite recent advances, particularly high-performance media that support 10 million cells per mL, culture medium development still relies on trial and error, according to Aziz Cayli, Ph.D., CEO of Cellca. The company specializes in cell-culture platform technologies, including its own CHO cell line specifically developed for large-scale manufacturing, and cell-culture media.
This is somewhat of a paradox. Due to safety and regulatory concerns, and the desire to standardize, modern media for large-scale production lack complex ingredients like serum and hydrolysates. Achieving the activity of these ingredients requires adding many more discrete compounds, particularly trace elements, that were present naturally in serum and hydrolysates.
The purity of modern reagents may be to blame at some level. Twenty years ago additives contained trace quantities of other substances that apparently benefited cells.
“Today, chemicals are nearly 100 percent pure, and we need to add those trace elements back,” Dr. Cayli observed. “Natural components contain a large number of chemicals. Eliminating one may remove from 10 to 50 individual components, including growth factors, trace elements, and lipids.”
Thus, the “magic dust” of several undefined ingredients gives way to many more scientifically characterized components.
An associated trend, ever-increasing cell density, has led to the use of feeds and supplements that also contain these nutrients. Off the shelf some of these preparations hold individual components at or near their solubility limits.
“We still don’t have a good understanding of what specific ingredients cells need to grow and produce,” Dr. Cayli said.
The challenges are to gain knowledge of cellular demand, and use it to tune in desired characteristics and performance. These objectives will require “a more rational approach to understanding factors affecting cell metabolism, growth, protein production and protein quality.”
Despite difficulties, rising biomass concentrations tell us that today’s cell culture media are much improved over those a decade ago. When hydrolysates or sera were widely used, cell counts ranged from about two to three million cells/mL.
“Today, by improving media quality, we have pushed viable cell concentrations to 30 million/mL in simple fed-batch cultures,” reported Dr. Cayli, adding that this is primarily due to media improvements.
Cellca has two goals in addition to raising biomass concentrations even further: suppressing metabolic waste (e.g., lactate), and improving product quality. Both objectives, Dr. Cayli believes, can be achieved by improving and fine-tuning media. One aspect of quality improvements is control over glycosylation. “Modern media contain as many as 70 components that may affect glycosylation.”
Recapitulating the art-and-science dichotomy, Thomas Noll, Ph.D., professor of cell-culture technology at the University of Bielefeld, noted that animal cell culture development itself was still “an empirical procedure. The application of proteomics and metabolomics remains hampered by the unavailability of a CHO protein database and, in case of metabolomics, by the cells’ mechanical instability and their compartmentalization.”
Dr. Noll’s group is developing new strategies for rational process development based on process characterization and identifying the influence of process conditions, at the cellular and molecular levels, through application of differential proteomic and (intracellular) metabolomic analysis. Proteomics and metabolomics allow the most direct measurement of a cell’s physiological activity and, according to Dr. Noll, “have proven their potential for microbial cell-line and process development.”
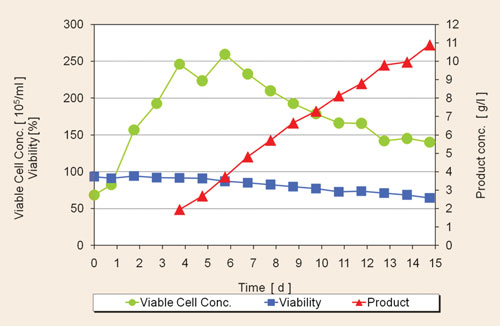
Vialble cell concentarion (per mL), cell viability (percent), and product concentration (g/L) for an optimized CHO cell culture [Cellca]
Improving Transfection
The production of recombinant proteins always involves either transient or stable transfection. The former is rapid but is not inheritable, while the latter results in low transfection. Integrating vector DNA into the genome of the host cell tends to be random in frequency and efficiency, and resulting productivity unpredictable due to the need for selection and screening for the optimized clone.
Hence the great interest in meganucleases, zinc finger nucleases, tag-and-exchange, and other techniques. One such alternative, scaffold/matrix attachment region (S/MAR) minicircle DNA, was the subject of a presentation by Bernd Rehberger, a scientist at Rentschler.
Minicircles are circular, superhelical DNA vectors resulting from homologous recombination of “normal” parental plasmids. Characterized by the absence of unwanted bacterial sequences and other potentially interfering elements originating from the parental plasmid, minicircle DNA is viewed as a way to achieve high stable transfection rates, particularly when combined with S/MAR elements.
According to Rehberger, whose company sells S/MAR minicircle reagents, minicircles result in transfection rates as high as 90%, with 1% of plasmids entering the genome.
The potential for generating stably transduced cell lines with more predictable productivity and growth behavior within a short time make the S/MA minicircle vectors an interesting alternative to conventional transfection since they eliminate the need for time-consuming selection and screening. Incorporation of the desired gene can be monitored using cell sorting based on green fluorescent protein.
Minicircles are also under investigation as gene-therapy agents and DNA vaccines, although Rehberger said “it will still take some years to achieve the levels of quality and reliability required for human therapeutics.”