November 1, 2016 (Vol. 36, No. 19)
In Hopes of Realizing the Clinical Promise of RNA Interference (RNAi), Scientists Are Identifying Novel Targets and Developing “Smart” Carriers
According to David Lewis, Ph.D., CSO, Arrowhead Pharmaceuticals, RNAi is translatable to the clinic, as demonstrated by proof-of-concept studies. RNAi is, in fact, emerging as a clinic-ready technology. This development must be encouraging to those hoping to subdue diseases that still manage to evade treatment, even though they present genetic targets.
Basic research has demonstrated repeatedly that RNAi is extremely efficient. “The technology is absolutely effective once it gets into the right cells,” explains Frank Slack, Ph.D., director, Institute for RNA Medicine, at Beth Israel Deaconess Medical Center. “And the effects of RNAi are long lasting, modifying gene expression for months.”
Moreover, RNAi therapy can impede the gene that causes disease, rather than a target further down the signaling pathway. RNAi is also not hindered by binding affinity or membrane localization, as are many of the currently available drug therapies (that is, small molecules and antibodies).
Most of the therapeutic applications of RNAi to date have been targeted specifically to the liver, due to this organ’s tendency to take up circulating molecules and accumulate RNAi triggers. Although clinical success in hepatocytes is an important pioneering achievement, the potential of RNAi lies in the technology’s versatility and precision. It can be used to modulate the expression of virtually any disease-causing gene, so long as it brings the right RNAi therapeutic to the right place.
Efforts to satisfy these conditions—the right therapeutic, the right target—are now underway. RNAi researchers are currently working to identify novel targets, particularly in noncoding RNA, as well as to expand delivery destinations, including hard-to-reach tissues.

Basic research has demonstrated repeatedly that RNAi is extremely efficient. RNAi therapy can impede the gene that causes disease, rather than a target further down the signaling pathway. [Poba / Getty Images]
Nanoparticle Formulation
Stephen Hart, Ph.D., professor of molecular genetics at the UCL Great Ormond Street Institute of Child Health, asserts that RNAi therapeutics may be delivered by means of “multifunctional nanoparticles.” His team is developing what it calls receptor-targeted nanocomplex (RTN) technology. So far, RTN technology has been evaluated for its ability to deliver short interfering RNA (siRNA) molecules to pediatric patients who suffer diseases such as cystic fibrosis and neuroblastoma.
RTN technology makes use of nanoparticles that not only target receptors on the intended cell types, but also release therapeutic nucleic acids directly to the cytoplasm. Essentially, these nanoparticles overcome the delivery barriers that have long frustrated RNAi therapeutics.
Expressions of enthusiasm for current RNAi-mediated therapies may be distinguished from the hype that accompanied the founding of the RNAi field. “The field has seen extensive improvements in technology,” says Dr. Hart. “Now we have a realistic assessment of how these therapies can be implemented successfully.”
Along with this resurgence in RNAi therapy comes hope for patients who have few or no other options available. In pediatric neuroblastoma, for example, if traditional treatments fail and the patient relapses, no other treatment is available, notes Dr. Hart. “Strategically designed nanoparticle delivery of gene-silencing siRNA,” he suggests, “provides some hope under dire circumstances.”

Targeted Liptide™ nanocomplexes bind and enter cells within tumors after entering the tumor though leaky blood vessels. Targeted and nontargeted Liptide nanoparticles were loaded with fluorescently labeled siRNA and administered to mice with xenograft tumors through the tail vein. Mice were killed 24 hours later; tumors were sectioned, nuclear stained with DAPI (blue), and analyzed by microscopy. Images show the targeted formulations (red) had entered cells by the EPR effect then bound and entered cells within the tumor, while nontargeted formulations, although within the tumor, were pooled extracellularly. The same pattern was observed in groups of n=3. [University College London]
Multifaceted Technology
Arrowhead Pharmaceuticals is also developing RNAi delivery systems. These systems, which make use of the company’s Dynamic Polyconjugate (DPC) platform, can target tissues outside the liver.
For example, one system targets malignant renal cells. It consists of a membrane-active polymer to promote RNAi trigger endosomal release, a ligand that binds to alphaV-containing integrins on tumor cells, reversible masking to prevent polymer activity prior to endosomal compartmentalization, and an RNAi trigger to hypoxia-inducible factor 2a. This system allows the polymer-RNA-ligand complex to stably circulate prior to uptake by the endosome, which is essential for successful delivery of the treatment.
This multifaceted approach is translatable to various diseases. “We are interested in developing RNAi delivery platforms that will ultimately enable plug-and-play incorporation of any ligand of interest,” explains Dr. Lewis. He envisions that RNAi and antisense oligonucleotide therapeutics will constitute a third modality in drug development.
“It’s a very exciting time,” Dr. Lewis continues. “As with antibodies, the field started out with unrealistic expectations and needed time to overcome challenges. But now we are starting to see real progress, and I anticipate that approved RNAi treatments will be available in two to three years.”
Subcutaneous Directed Delivery
Clinical administration of therapeutic siRNA is also showing marked advances. Alnylam Pharmaceuticals is currently conducting clinical trials with its GalNAc-siRNA conjugate technology. The GalNAc ligand is attached to a chemically modified siRNA and is taken up by the asialoglycoprotein receptor (ASGPR) expressed on the hepatocyte cell membrane. Importantly, this GalNAc-siRNA conjugate technology allows for subcutaneous administration, considerably increasing its clinical applicability and therapeutic potential for a range of diseases.
“The simplicity of the platform is what holds the greatest potential,” says Tracy S. Zimmermann, Ph.D., director of research at Alnylam. “We have achieved efficient, subcutaneous delivery that results in potent and durable RNAi-mediated silencing of hepatic targets, possibly requiring only a single monthly dose for patients.”
This approach has clear benefits for developing investigational RNAi therapeutics for liver-expressed disease targets. It is also driving directed delivery research for other applications in the RNAi field.
“In less than 10 years, we have seen RNAi technology transform from a highly touted in vitro application to tangible, clinical success,” states Dr. Zimmermann. By 2020, Alnylam anticipates having 10 programs in advanced development and therapeutics on the market. This accelerated trajectory marks a huge accomplishment for RNAi technology.

Conjugates of small interfering RNA (siRNA) with asialoglycoprotein receptor ligand derived from N-acetylgalactosamine (GalNAc) have been pioneered by Alnylam Pharmaceuticals as a means to deliver any desired siRNA sequence to hepatocytes. For example, the siRNA-GalNac conjugate shown in this image has allowed for efficient knockdown of genes in the human liver.
Humanizing Discovery
Focusing on modulation transcription via microRNAs (miRNAs), researchers are aiming to recapitulate a more physiological approach to RNAi. “Gene knockdown is not a very physiological process,” explains Kai C. Sonntag, M.D., Ph.D., assistant professor of psychiatry, Harvard Medical School. As researchers are beginning to understand more about these micro-nucleic acids, it is clear that mammalian cells, in particular, rely on miRNAs to judiciously modulate gene transcription rather than on the more absolute knockdown achieved with siRNAs.
Dr. Sonntag and his colleagues are meticulously profiling postmortem human brain tissue from patients with diseases such as Alzheimer’s, Parkinson’s, and schizophrenia. Employing laser capture microdissection techniques, the researchers isolate a very specific pool of neurons (dopaminergic neurons from the substantia nigra and pyramidal neurons from the cortex, for example) for analysis by microarray and, more recently, RNA sequencing.
With this methodical approach, Dr. Sonntag’s team identifies miRNAs that are up- or downregulated in diseased patients. The team compares these targets to the regulatory transcription profile for focused translation into experimental systems.
Dr. Sonntag has identified miR126, a known insulin regulator in other cell types, as potentially important in neurodegenerative disease. (There is substantive literature implicating insulin signaling in Alzheimer’s.) Increased miR126 prompts vulnerability of cultured cells to various neurotoxins (amyloid-β, staurosporin, and 6-hydroxy-dopamine).
The team also showed that this increased sensitivity could not be abrogated by neuroprotective factors such as insulin-like growth factor 1, nerve growth factor, brain-derived neurotrophic factor, and soluble APPα. Moreover, inhibition of miR126 was sufficient to reverse the neurotoxic effect.
Although Dr. Sonntag’s group is primarily focused on preclinical work—screening human material, identifying mechanisms, and verifying these experimentally in the laboratory—the potential for therapeutic application is high.
Dr. Sonntag stresses the importance of identifying these miRNA targets in human material. “It is essential when studying human diseases like Alzheimer’s.” The literature is filled with studies conducted in animals but, in fact, those complex brain diseases are not fully recapitulated in any animal model, and challenges translating basic research to the clinic have shown that.
“Billions of dollars have been spent to target amyloid-β and tau in Alzheimer’s, for example, with very limited therapeutic success,” observes Dr. Sonntag. “Maybe we need to rethink the pathogenesis of Alzheimer’s. We must appreciate what the human material is telling us.”
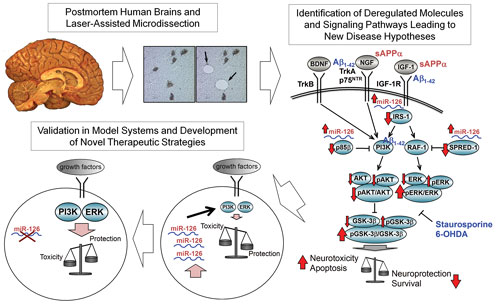
To develop new therapies for neurological disorders, a Harvard Medical School team is dissecting postmortem human brains, analyzing molecular pathways, and working with various model systems. For example, as summarized in this image, the team identified miR-126 as a potential therapeutic target for brain disorders. Elevated levels of miR-126 increase the vulnerability of neurons, but inhibiting the miRNA is neuroprotective without affecting normal cell function.
Addressing OncomiR Addiction
Dr. Slack is also focused on harnessing the power of noncoding RNAs as a means of generating better therapeutics for diseases that are exceedingly difficult to treat. He describes his efforts to target lymphoma tumor cells by identifying miRNAs that are mis-expressed in human cancer samples and then functionally validating these targets.
Delivery remains the focus for miRNA therapeutics as it has been for the broader RNAi field. Although the field has dragged because of this, Dr. Slack asserts that the “doom and gloom around this issue is lifting” as successes pile up. “Now we just need to identify what is unique about where you want to target the siRNA, and then there are ways to get it there.”
Borrowing heavily from predecessors in siRNA and also antisense oligonucleotide technology, Dr. Slack is seeing success conjugating chemical moieties onto anti-miRNAs, successfully targeting them to lymphoma tumors in animal models.
Adding a unique “twist” to existing strategies, Dr. Slack has teamed up with collaborators who were focused on penetrating the highly acidic tumor microenvironment with a pH-sensitive peptide for imaging purposes. The peptide targets anti-miRNA to tumor cells, effectively crosses the membrane and engages miRNAs in the cytoplasm directly, circumventing endosomal interaction that has proved troublesome in other studies.
As for the efficacy, mice receiving miR-155-modulating treatment saw decreased tumor size after only a few days. miR-155 is a strong candidate for targeted RNAi as overexpression causes tumors in mice. Furthermore, the tumors appear to be “addicted” to expression of miR-155.
The idea that tumors rely completely on a single oncomiR for growth and survival, so-called “oncomiR addiction,” is reminiscent of “oncogene addiction,” which has been documented in various cancers and employed successfully for identifying strategic targets in drug development. And Dr. Slack has demonstrated that miR-155 is not only oncogenic, but also sensitive to inhibition, opening up the therapeutic prospects for anti-cancer therapy. In fact, MiRagen Therapeutics recently launched a clinical trial investigating how miR-155 interference translates to humans.
Although a successful trial has yet to come to fruition for miRNA-interfering technology, the technical hurdles are being overcome and excitement is growing again. “We need one hit molecule,” insists Dr. Slack. “After one big success, things will take off.
“It’s a very exciting time. The field is maturing, and the skepticism around whether noncoding RNAs are going to do anything has lifted completely. People are paying attention now to determine how they can really be useful.”



