Ensuring we and generations to come have enough to eat in the face of climate change and consequent species migrations, involves the necessary evil of killing insects that destroy our crops. Conventional, environmentally detrimental chemical pesticides are species-agnostic and often kill harmless insects and progressively decline in efficacy as pests evolve resistance. Technologies that selectively control or kill pest species offer a safer option.
One such invasive insect pest is Drosophila suzukii, which poses a threat to agricultural yields, particularly to the production of fruits such as berries, cherries, plums, and grapes, in western countries. Thus far the impact of control measures to limit the spread of D. suzukii has been less than optimal.
An article published in GEN Biotechnology (“Precision Guided Sterile Males Suppress Populations of an Invasive Crop Pest“) reports the development of a programmable CRISPR-based method that if deployed at scale in the wild, could potentially replace fertile male D. suzukii with sterile counterparts, thereby effectively, specifically, and safely curbing this pest population. Although developed on D. suzukii, the authors claim that the method can be modified to target other pest species, precluding the need for environmentally unfriendly pesticides.

The study, conducted by scientists at the University of California, San Diego (UCSD), including co-lead authors Nikolay Kandul, PhD, Junru Liu, PhD, and Anna Buchman, PhD, and corresponding author, Omar Akbari, PhD, uses a temperature-inducible, precision guided, sterile insect technique (pgSIT), reported by the team in an earlier study, to generate infertile, but fit and competitive D. suzukii males with slightly reduced lifespans. Through empirical experimentation and mathematical modeling, the researchers showed that repeated release of sterilized males can rapidly and successfully curb or eliminate D. suzukii populations. (Akbari’s team has used pgSIT in an earlier study to sterilize mosquitoes).
“It’s a safe, evolutionary stable system,” said Akbari, a professor in the School of Biological Sciences’ department of cell and developmental biology at UCSD. “The system does not lead to uncontrolled spread nor does it persist in the environment—both important safety features that will help it gain approvals for use.”
“This builds off work previously published,” said Rodolphe Barrangou, PhD, editor of The CRISPR Journal. “The study illustrates how next-generation CRISPR-based gene drives can be used to control crop pests. The data convincingly show that this approach has technical benefits and enables population suppression through deployment of competitive sterile males. Importantly, this proof-of-concept study provides a basis to ponder commercial applications.”
The pgSIT system edits essential genes that determine sex and fertility. The authors used two transgenic D. suzukii strains, one expressing Cas9 in germline and somatic cells and the other expressing guide RNAs targeting genes essential for female survival and male fertility, such as sex lethal (Sxl), double sex (dsx), and transformer (tra). The technique produced up to 100% sterile males with females either killed or turned into intersex flies.
The authors showed that the pgSIT system was effective when pgSIT sterile males are released at ratios that are comparable to approaches that use conditional female lethality strains, and are lower than the release ratios recommended for conventional SIT (sterile insect technique) paradigms. In addition, conventional SIT programs use DNA-damaging agents, such as ionizing radiation, to generate sterilized males. This limits fitness and mating competitiveness of released males.
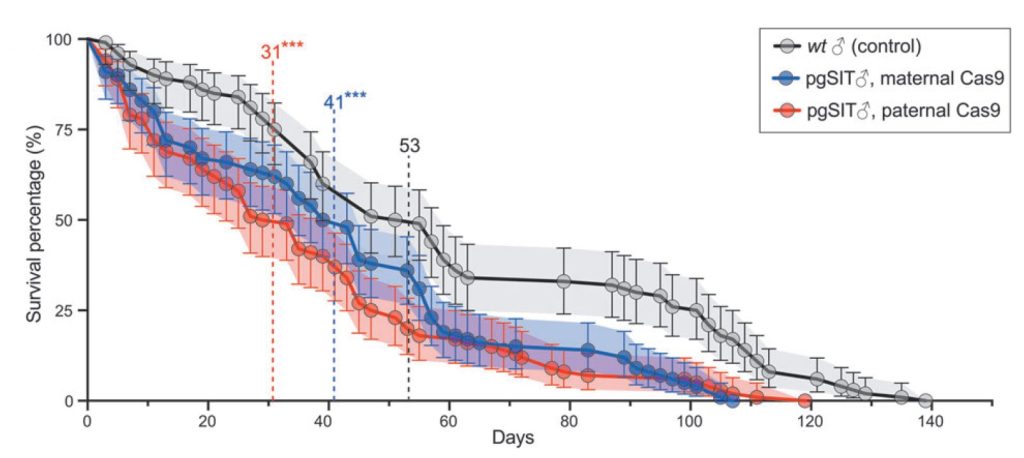
The authors showed combining pgSIT D. suzukii with wildtype males significantly decreased the egg hatching rate, indicating that the sterile males are competitive and successfully compete with wildtype males to mate with females. The authors also showed that increasing release ratios of pgSIT males to wildtype males increased the rate of population decline.
”The pgSIT technique is an attractive way to control D. suzukii populations,” said Kutubuddin Molla, PhD, a scientist at the National Rice Research Institute in Cuttack, India, and an editor at the The Plant Cell journal. “However, before environmental release, we must be careful to avoid any anticipated risk and unintended ecological consequences. If we could develop a recall system of gene drive, we would have more confidence of environmental release of gene-drive insects.”
Barrangou concurred. “We have to be mindful and cautious about practical deployment and ensure that proper studies are carried out to assess release safety and ecological impact and consequences,” he said. “Depending on release scale and pattern, population reduction and control rather than elimination per se is presumably the outcome.”
Akbari said that his team has developed pgSIT for several species in the past four years, and hopes to use it as a platform technology to safely control the spread of other pests in the real world.


