August 1, 2018 (Vol. 38, No. 14)
MilliporeSigma Describes How to Carry Out Operations with Robust and Turnkey Platforms
Development of an industry-scale bioproduction process is a time-consuming, multistep operation that requires extensive biological and engineering expertise. Although the generation of unique recombinant cell lines hinders development of standard platforms, reduction of variables in cell lines, cell culture media, and growth vessels permits common strategies to achieve rapid and robust results.
A holistic upstream solution can help address this challenge. In our turnkey and complete solution, we’ve reduced the number of variables by integrating multiple product lines, optimizing the process, and providing detailed protocols to guide users to a path of successful clone generation, process development, and scale-up while reducing resource requirements and timelines.
Developing a robust bioprocess introduces many challenges for the user—the most significant being time and resources. Due to complex biological properties, development of a recombinant cell line requires working with a large number of cells and isolating a so-called “needle in a haystack.”
This process requires maintenance of numerous cell cultures a few times each week. After a cell line is isolated and selected, development of a robust bioreactor process requires multiple two-to-three-week-long runs at multiple scales—from small scale to pilot and production scale. These systems require daily sampling and extensive analysis to determine methods to improve the process for future runs.
Two other challenges are process and product quality. Current process economics and efficacy needs dictate expected product titer and quality benchmarks at large production scale. Simply running the process does not guarantee that these attributes will be obtained. Alternatively, developing a basic platform into a system that can be utilized at scale for multiple molecules may take years.
Recombinant Cell Line Development
The CHOZN® platform is a recombinant CHO (Chinese Hamster Ovary) mammalian cell expression system for the fast and easy selection and scale-up of clones producing high levels of recombinant proteins. It is supported by proven protocols, paired media and feed, as well as customized services for cell line development and cell line engineering.
The host cell line is GMP-banked, and the history of the cell line is traceable and documented to support regulatory filings. The expression vector is IP-free, and the cell culture media is produced under strict GMP guidelines to support all regulatory filings. A description of the workflow and all the protocols needed for successful clone generation is described in the CHOZN technical filing.
Results from four independent cell line development projects are shown in Figures 1A & 1B. Cells were transfected with different dual-expression vectors containing light and heavy antibody genes. After selection, screening, and isolation, the final fed-batch titers for the best clones of each of the four projects are shown. Titers range from 2 g/L to over 4 g/L (Figure 1A).
This range of titers is expected due to clone variability regarding molecule expressability, cell growth potential, and other biological factors. The corresponding specific productivity results for these clones are shown in Figure 1B. Metrics such as these can aid in selection for specific applications (i.e., fed-batch or perfusion).
All potential production clones should be evaluated for recombinant protein expression stability in a three-month study. In the study shown in Figure 1C, the 10 highest expressing clones from a cell line development project were passaged twice a week for approximately three months, and different linages were evaluated for titer in fed-batch.
The percentages of retained titer are shown in Figure 1C. Eight of the 10 clones retained at least 75% of the initial fed–batch titer. Collectively, these studies demonstrate that the CHOZN GS platform is a robust solution for obtaining high titers and stable recombinant protein expression.
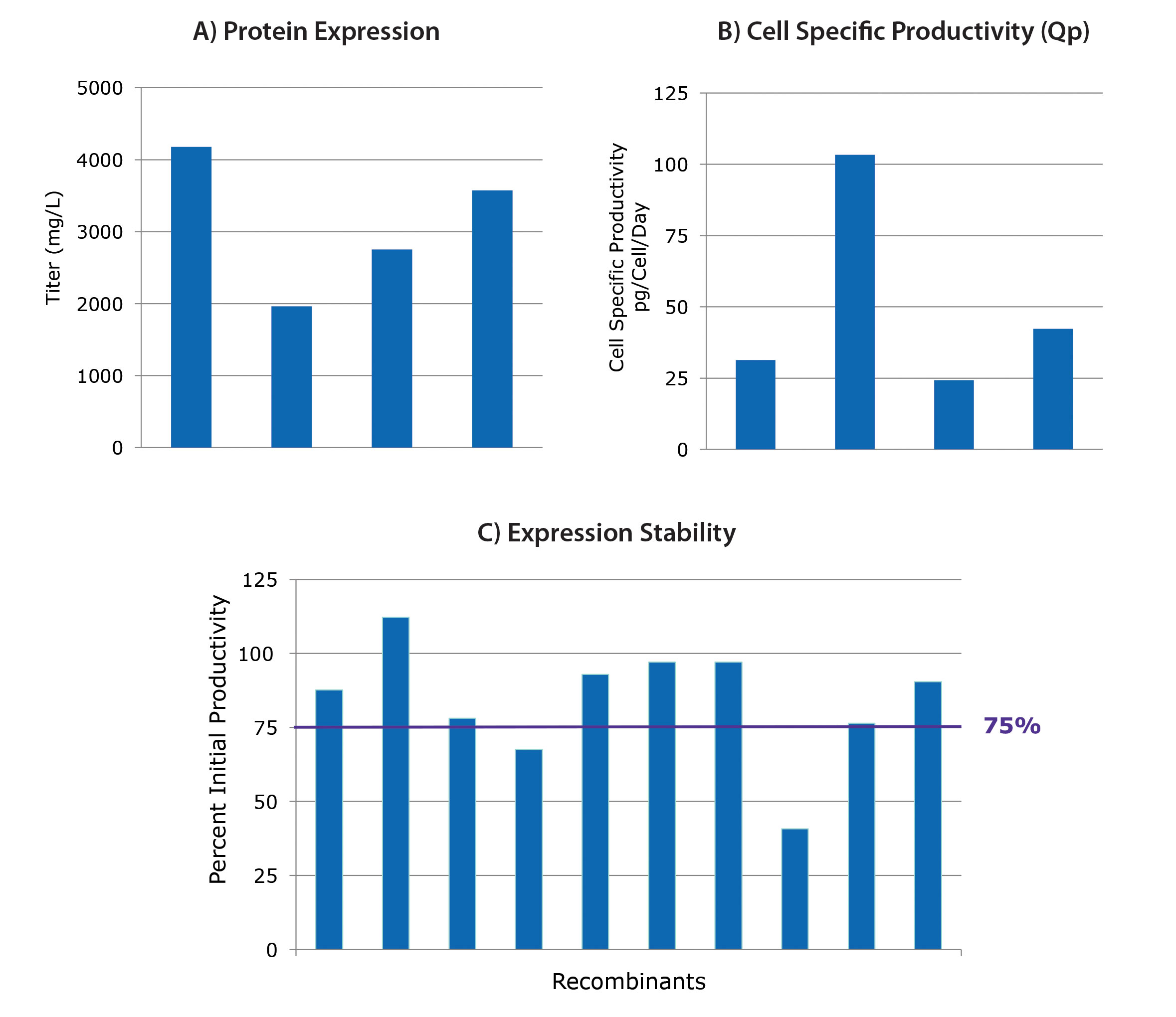
Figure 1. Performance of CHOZN® GS clones. The CHOZN GS-/- cell line has been modified to eliminate endogenous glutamine synthetase (GS). Titers (A) and productivity results (B) from four different cell line development projects are shown. Percentages of retained titers (C) are shown for the 10 highest expressing clones from a cell line development project.
Optimizing Cell Culture Media and Feed Strategy
For this turnkey solution, growth and production metrics can be improved using matched cell culture media and by varying feed amounts and timing. Our platform utilizes EX-CELL® Advanced cell culture media and feeds. In the example shown in Figure 2, a CHOZN GS recombinant cell line was subjected to feed optimization by varying feed amounts and timing as indicated.
Compared to the control, one of the EX-CELL Advanced feed regimens improved titers 30%. When these or similar strategies are used, improvements can be expected with CHOZN GS recombinant cell lines.
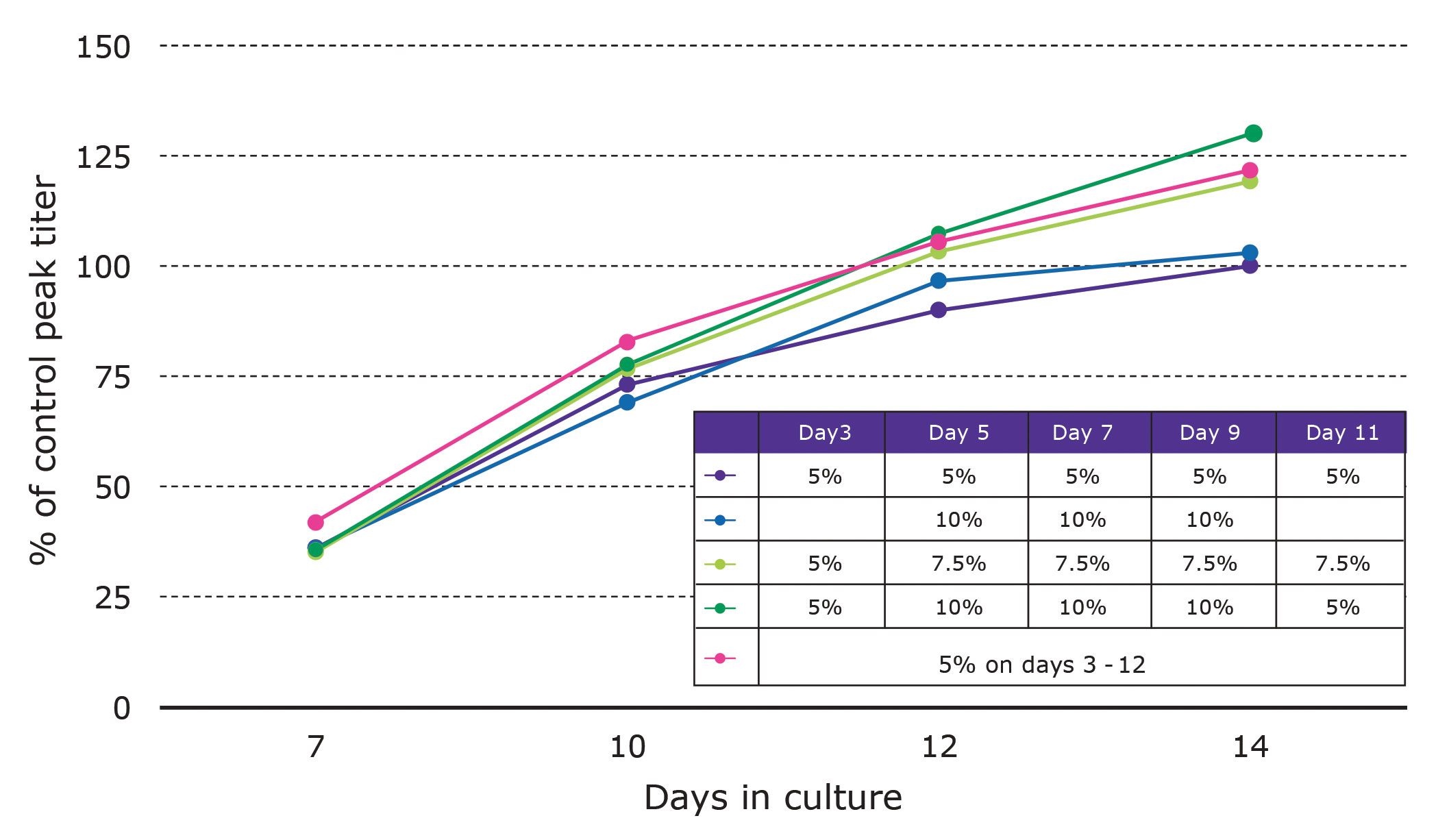
Figure 2. EX-CELL® advanced cell culture feed optimization. Compared to the control, one of the regimens improved tiers 30%.
Scale-Up
The performance of a recombinant cell line at large scale is a key parameter for upstream success. To ensure that performance metrics such as titer and quality are met, an understanding of small-scale models and scaling parameters for a particular system is needed. We’ve built a complete portfolio of scalable, single-use bioreactors based on these factors to improve the speed to upstream success.
The small-scale Mobius® 3L single-use bioreactor contains three probe ports, all the connections needed for both gas and feed additions, and a sampling port. The system comes presterilized and ready to connect to a variety of controllers.
The pilot-scale Mobius 50- and 200-L bioreactors contain stainless steel bases and integrated controllers. A presterilized single-use bioreactor bag needs to be installed into the stainless-steel carrier with a SensorReady loop prior to running the system. All of the connections are made using our Lynx S2S single-use sterile connector.
Our production-scale Mobius 1000- and 2000-L bioreactors have the same features as our 50- and 200-L systems, with the added benefit of a vessel drawer, allowing the easy installation and removal of single-use assemblies.
Keeping the importance of speed in mind, we set out to scale-up from a shake flask to the 50-L scale (Figure 3A). Duplicate shake flasks were run in parallel with the feed strategy optimized in small scale. Three duplicate runs of the 3-L system were performed with the agitation at 20 Watt/m3, the pH at 6.9, the dissolved oxygen (DO) at 30%, and the temperature 36.8 °C.
In the first run, we learned which sparging strategies worked best for these cells and determined the power setting. In the second run, we determined how to manage the pH set point of the bioreactor. In the third run, we scaled up to the 50-L system.
In addition to speed and matching product titers, matched product quality is paramount for upstream success. Our platform was designed with product quality in mind. In the experiment above, antibodies were collected at harvest and the glycan species were identified.
The percentages of the four major glycans (G0 in dark blue, G1F in turquoise, G2F in purple, and high-mannose in blue-green) for each of the scales are shown in Figure 3B. Across the scales, the percentages are highly similar.
These results show that the CHOZN GS cell line is scalable for titer and product quality at 3-L and 50-L scale with limited optimization, resources, and time.
In conclusion, the speed of cell line and process development is a key driver in biologic production. We presented a turnkey solution that was built with speed in mind. The platform not only provides high titer, but also provides a path to scalable critical quality attributes. This solution provides users confidence of upstream development success.
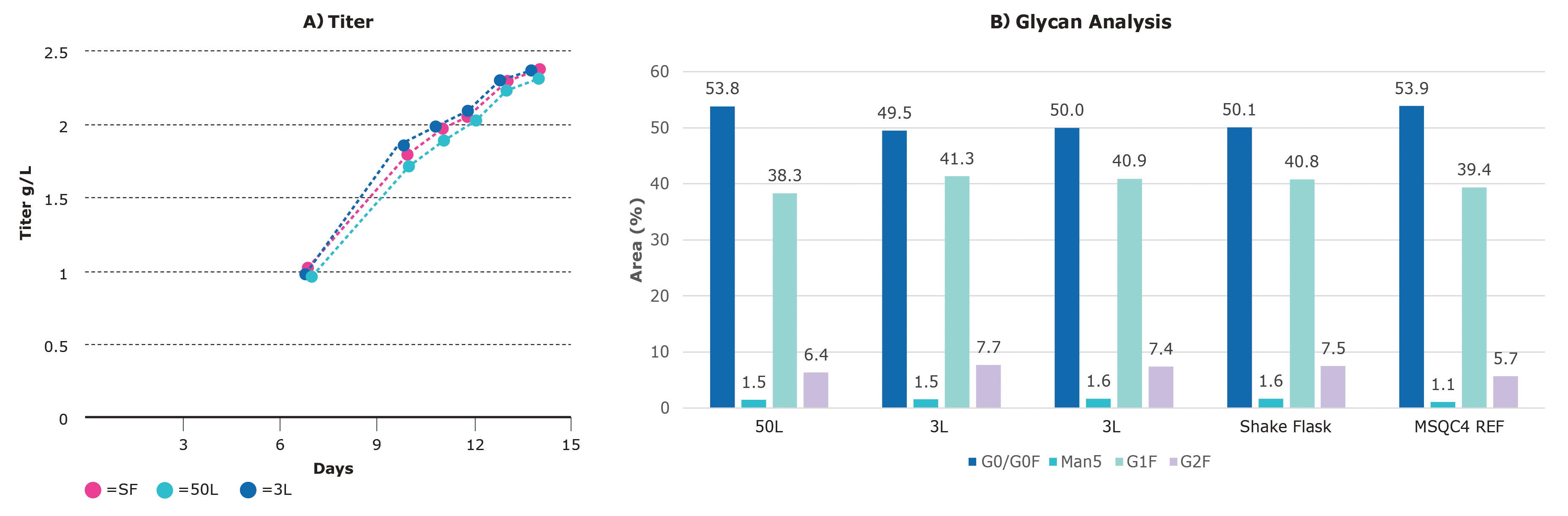
Figure 3. Scalability of a CHOZN® recombinant cell line in Mobius bioreactors. (A) At all the scales evaluated—shake flask (SF), 3 L, and 50 L—the titer results in the bioreactors were similar and peaked around 2.3 g/L. (B) Glycan analysis identified and quantified the major glycan species.
Joe S. Orlando, Ph.D., is principal scientist at MilliporeSigma.



