May 1, 2015 (Vol. 35, No. 9)
All Is Not Quiet On the Immunological Front
Overseeing immune surveillance. Driving cellular responses to changing environmental conditions. Maintaining homeostasis. These tasks are accomplished thanks to the complex network of intra- and intercellular communications.
No wonder the field of cell signaling continues to grow and expand, as does its vocabulary. Inflammasome, signalosome, supramolecular organizing centers, and other immune system signaling terms are working their way into everyday signaling language.
The latest cell signaling advances will be discussed June 7–12 at a meeting organized by the Federation of American Societies for Experimental Biology (FASEB). The meeting, “Signal Transduction in the Immune System,” will take place in Big Sky, MT. It will cover topics such as lymphocyte activation and development, imaging of cell activation and differentiation, immune deficiencies, cytoskeletal changes that regulate leukocyte activation and migration, and innate lymphoid cells.
Innate lymphoid cells include natural killer (NK) cells, which play key roles in innate immunity by fending off viruses and eliminating cancer cells. As the FASEB meeting’s participants will make clear, NK cells also influence the antigen-specific immune responses of other important members of the community, such as dendritic and T cells.
NK cells are controlled by both activating and inhibitory receptors. When activating signals predominate, NK cells kill target cells primarily through natural cytotoxic responses. They also secrete into the local environment cytokines such as interferon gamma (IFNγ), which then serve to further amplify the immune responses of other cells.
André Veillette, M.D., professor, division of experimental medicine, University of Montreal, studies receptor-driven signaling pathways that control NK cell functions. He leads a team of scientists that has been investigating mechanisms for NK cell-mediated killing of abnormal hematopoietic (blood) cells.
“We studied the signaling lymphocytic activation molecule (SLAM)-associated protein (SAP) family that consists of a group of intracellular adaptor proteins,” says Dr. Veillette. “In humans, two members include SAP and Ewing’s sarcoma-associated transcript 2 (EAT-2). SAP is an essential component promoting NK cell killing of abnormal blood cells, such as is found in leukemia and lymphoma. Its interaction with SLAM receptors is critical to this process.”
“Little was known about how EAT-2 functions,” he continues. “We wanted to better understand the cooperation between EAT-2 and SAP.”
Using a variety of genetic, biochemical, and imaging strategies, the team identified the molecular chain of events driving this pathway. They found that EAT-2 and SAP perform very different internal functions using distinct mechanisms of action.
“Our studies demonstrated that EAT-2 works by linking SLAM family receptors to phospholipase Cγ, calcium fluxes, and Erk kinase,” Dr. Veillette explains. “Unlike SAP, it also accelerates polarization and exocytosis of cytotoxic granules aimed at target cells.”
According to Dr. Veillette, since these intracellular adapter molecules are linked to the cell surface receptor SLAM family, they make attractive therapeutic targets.
“For example, Bristol-Myers Squibb is utilizing a SLAM family humanized monoclonal antibody, elotuzumab, in Phase III clinical studies against multiple myeloma,” Dr. Veillette notes.
“In May 2014, it was granted Breakthrough Therapy designation by the FDA. Our future work will focus on better understanding such receptors and how they are regulated to help identify other potential new treatments for blood cancers.”
Eluding NK Surveillance
Studies demonstrating that the use of cytokines can enhance immune responses against tumors opened up the possibility of using them therapeutically. For example, clinical trials treating advanced melanoma and renal carcinoma with high doses of IL-2 resulted in substantial improvements in a small fraction of patients. Many of the cytokines utilized in clinical trials are capable of activating NK cells and may promote antitumor activity of these cells.
NK-mediated recognition and rejection of tumors is being researched by David H. Raulet, Ph.D., a professor of immunology and pathogenesis at the University of California, Berkeley. He is focused on how regulating NK cells can be of benefit in cancer therapeutics.
“The effectiveness of NK cells depends on their ability to attack infected or malignant cells while maintaining self-tolerance,” maintains Dr. Raulet. “Their adaptability to their environment is affected by microenvironmental cues and by the information they receive from host MHC class I molecules. The latter operate via interaction with NK cell inhibitory receptors of the KIR (killer inhibitor receptor) family in humans, and Ly49 molecules in mice (and CD94/NKG2A dimers in both).”
Tumorigenesis is often associated with down-modulation of MHC class I molecules on tumor cells, thus priming them for elimination by NK cells. However, often advanced tumor cells are deficient in MHC class I expression but still manage to evade the immune responses by NK cells by unknown mechanisms.
A postdoc in Raulet’s laboratory, Michele Ardolino, Ph.D., tested whether treatment with cytokines known to activate NK cells could enhance therapeutic treatment in tumor-bearing mice via NK cell activation. “We found substantial therapeutic benefit in mice having MHC class I-deficient tumors that were treated with a combination of IL-12 and IL-18 or with an IL-2 mutant (H9 “superkine”) that functions independently of the native/normal IL-2 receptor,” notes Dr. Ardolino. “These treatments were not effective in mice that had tumors bearing MHC class I molecules.”
Dr. Raulet surmises that the cytokine efficacy was dependent on reversal of the anergic state of the NK cells in the MHC-deficient tumor-bearing mice: “The anergy of the NK cell was associated with impairments in early signal transduction of activating receptors in NK cells. These results demonstrate that MHC class I-deficient tumor cells can escape immune surveillance by functionally inactivating NK cells. They further suggest that specific cytokine therapies may be highly beneficial as a means of enhancing NK cell functions in cancer patients, but mainly in patients where NK cells have been inactivated by tumor cells.
Super-Resolution Marvels
In 1873, the German physicist Ernst Abbe determined that the resolution of optical imaging instruments, such as microscopes and telescopes, was fundamentally limited by the diffraction of light. New advances in microscopy have overcome this previously insurmountable barrier, and it is now possible to peer into the inner life of cells and their organelles.
One of these advances garnered a Nobel Prize in 2014. The award went to three scientists (Eric Betzig, Stefan Hell, and William Moerner) who developed super-resolution microscopy. This technique exploits fluorescent molecules to reveal the nanoscale features of cell membranes, intracellular organelles, and protein superstructures.
Super-resolution imaging is already being used to pursue subjects of immunological interest, such as NK cell activation and the interaction of these white blood cells with tumor cells. Investigators visualizing these mechanisms include Daniel M. Davis, Ph.D., a professor of immunology at the University of Manchester. (Dr. Davis is perhaps more widely known as the author of The Compatibility Gene, a popular science book.)
“There are many reasons for utilizing super-resolution microscopy, but the basic one is just that you can watch cells with unprecedented resolution while they integrate signals and decide whether or not to respond,” says Dr. Davis. “For example, by observing the interaction of an NK cell with proteins that are normally found only on tumor cells, one can see how the NK cell responds, and even observe unexpected phenomena, without a preformed hypothesis.”
In one study, Dr. Davis and colleagues wanted to determine how immune cells expressing germline receptors for viral protein are able to differentiate between virus-infected cells and viral particles. A different immune response must be directed against virally infected cells rather than the particles. NK cells secrete lytic granules that kill virus-infected or transformed cells, and they secrete cytokines to communicate with other cells.
Integration of signals between activating and inhibitory receptors on NK cells are driven by reactions to such target cells. This occurs across a structured interface called the immune synapse, which Dr. Davis co-discovered in the late 1990s.
“We used super-resolution microscopy to follow the movements of F-actin, lytic granules, and interferon-γ in primary human NK cells under different conditions,” states Dr. Davis. “We found that NK cells can recognize influenza virus particles but that the particles were not sufficient stimulus to open up the actin mesh. This happened only if the integrin LFA-1 was co-ligated.
“These results suggest that integrin recognition can help NK cells differentiate between free pathogens and pathogen-infected cells. But the opening up of the actin meshwork could be detected only with super-resolution microscopy.”
Dr. Davis’ studies demonstrate how cracking the limits of diffraction can open a whole new world of visualizing cellular events—even those that occur in confines smaller than 100 nanometers.
“The human body is one of the greatest wonders of the universe,” observes Dr. Davis. “Its complexity, delicacy, and elegance are particularly illustrated in how our immune system works.”

A super-resolution fluorescence image of cortical actin in NK cells. The image, provided by Daniel M. Davis, Ph.D., of the University of Manchester, is color coded. Red indicates where the periodicity of the cortical actin mesh at the synapse increased the most in specific domains when the NK cell was activated.
Innate Sensing Pathways
For more than a century, the immune stimulatory effects of DNA have been recognized. “Detection of foreign DNA, such as from an invading microbe, is a fundamental mechanism of host defense,” notes Zhijian “James” Chen, Ph.D., a professor of molecular biology at the University of Texas Southwestern Medical Center, and an investigator of the Howard Hughes Medical Institute.
“We are studying the mechanisms underlying detection of these danger signals and how they trigger the host innate immune response such as expression of type I interferons.”
Dr. Chen and colleagues recently identified cyclic guanosine monophosphate-adenosine monophosphate (cGAMP) synthase (cGAS) as a sensor of cytosolic DNA that triggers the production of inflammatory cytokines and type I interferons.
“cGAS binds generically to any DNA, and it catalyzes the synthesis of a specific cGAMP isomer that functions as a second messenger for the binding and activation of the adapter protein STING,” asserts Dr. Chen. “Once the pathway is engaged, STING activates two protein kinases (IκB kinase and TANK-binding kinase 1) that lead to induction of interferons and cytokines.”
To better understand the function of cGAS in vivo, Dr. Chen’s team generated a cGas knockout mouse strain. They found that cells from cGAS-deficient mice, including fibroblasts, macrophages, and dendritic cells, could not produce type I interferons and other cytokines following DNA virus infection or DNA transfection.
The knockout mice could not defend against infection by herpes simplex virus 1 as compared to wild-type mice. “Thus, cGAS is a general DNA sensor that activates the STING pathway,” says Dr. Chen.
Another finding by Dr. Chen’s group related to cGAS-activated synthesis of a specific cGAMP isomer, termed 2′3′cGAMP, the specific messenger that binds to STING. They determined that cGAMP is an effective adjuvant that can boost production of antigen-specific antibodies and T-cell responses in mice.
Dr. Chen states that although bacterial second messengers cyclic di-GMP and cyclic di-AMP are being studied for their potential use as vaccine adjuvants, 2′3′cGAMP is a much more potent ligand of STING: “It is possible that 2′3′cGAMP could be developed as an adjuvant for next-generation vaccines to treat infections and cancer.”
Inflammasomes as Microbial Sensors
The innate immune system provides the first line of defense against invading pathogens. Host pattern-recognition receptors (PRRs) recognize unique microbial signatures and engage signal transduction pathways to combat infection.
One of the major activation pathways is the inflammasome pathway. The inflammasome, a multicomponent protein complex, activates the caspase-1 cascade, which leads to maturation of the proinflammatory cytokines interleukin 1β (IL-1β) and IL-18. It also induces pyroptosis, a form of caspase-dependent programmed cell death in which an infected immune cell produces cytokines, swells, and bursts, attracting still more cells to repeat the inflammatory cycle.
Gabriel Núñez, M.D., a professor of pathology at the University of Michigan Medical School, is interested in understanding the mechanisms of PRRs including Nod-like receptors (NLRs).
“Of the four identified inflammasomes, three (NLRP1, NLRP3, and NLRP4) are activated by members of the NLR family,” details Dr. Núñez. “The NLRs include an amino-terminal caspase-recruitment domain (CARD), pyrin domain, acidic transactivating domain, a central nucleotide-binding-and-oligomerization domain (NOD) that mediates self-oligomerization, and a carboxy-terminal leucine-rich repeat believed to sense different microbes and endogenous damage.”
After pathogens infect a cell, NLR proteins sense the presence of released microbial ligands in the cytoplasm. For example, the release of pore-forming toxins by Staphylococcus aureus, Vibrio cholera, and Streptococcus pyogenes activates the NLRP3 inflammasome.
“This inflammasome has received much attention because it also has been linked to pathogenesis of autoinflammatory syndromes and a variety of inflammatory diseases,” remarks Dr. Núñez.
A number of important questions remain about how inflammasomes are involved in both health and disease. According to Dr. Núñez, these questions will be resolved only if scientists secure a more basic understanding of the biology and mechanisms of inflammasome activation.
“[We need] to know to what extent inflammasomes are involved in widespread conditions such as diabetes and atherosclerosis. We also need to determine how inflammasomes interact with other signaling pathways to orchestrate innate and adaptive immune responses,” explains Dr. Núñez. “As we gain more information about inflammasomes, we may be able to design and develop small molecules to block or delay these inflammatory diseases.”
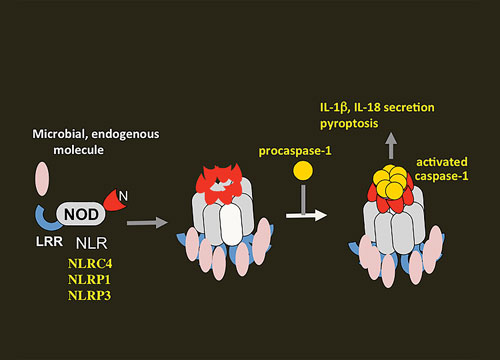
Activation and assembly of the inflammasomes via Nod-like receptors (NLRs) in response to microbial and endogenous stimuli. This image, provided by Naohiro Inohara, Ph.D., of the University of Michigan, shows how oligomerization of NLRs induces the proximity and activation of caspase-1.
Signalosomes and SMOCs
More than 100 years ago, Paul Ehrlich hypothesized that receptors were cellular communicators that were capable of recognizing distinct chemical structures such as toxins. We now know that receptors are sophisticated sensing components involved in multiple signaling pathways, and that they are even more complex than previously imagined.
Scientists today are investigating the molecular mechanisms underlying signal transduction by immune receptors. One such scientist is Hao Wu, Ph.D., professor of biological chemistry and molecular pharmacology at Harvard Medical School. She notes that the binding of ligands induces conformational changes in receptors, and she adds that such binding is often thought to involve the creation of receptor dimers and trimers that subsequently activate downstream signaling cascades. This traditional view, however, may be incomplete.
“We discovered a new scenario while we were working on crystallizing receptors,” Dr. Wu reports. “During their isolation, we found there were other proteins co-purifying. At first we thought this was an artifact, but then we realized these were real assemblies.”
Dr. Wu asserts that his team conducted structural studies that uncovered a vast network of higher-order signaling machines, or signalosomes: “We found that these higher-order complexes formed by helical symmetry and that they served to induce the activation of enzymes such as caspases, kinases, and ubiquitin ligases that then lead to cell death, cytokine maturation, and a host of inflammatory responses.”
Although it was known that receptors assemble into defined oligomers (such as occurs during apoptosis), the discovery of large and virtually “infinite” assemblies that have acquired signaling capabilities was novel.
“These signaling machines provide unique mechanisms to impart threshold responses and a means for temporal and more spatial control of signaling,” Dr. Wu emphasizes. “Further, this opens the door to suggest higher order assemblies may be an important aspect of many other biological processes since they promote the formation of very precisely ordered constituents that were initially present in low concentration of inactive states.”
According to Dr. Wu, additional studies further expand the new signaling paradigm: “On the basis of structural and cellular studies, we now believe there are supramolecular organizing centers (SMOCs) that are assembled on various membrane-bound organelles such as the actin network. These serve to increase local concentrations of signaling components to amplify what would otherwise be weak interactions.”
Overall, these studies provide a new way of thinking about immune network signaling. Besides being widely used in signal transduction in the immune system, higher-order signaling may characterize a general mechanism used by cells for numerous other biological functions. “This is such an elegant mechanism, why wouldn’t other cells follow it? Certainly, we have only begun to see the tip of the signaling iceberg,” concludes Dr. Wu.


