When a mosquito bite threatens to transmit infection, induce disease, or even contribute to early death, as it often does in malaria-endemic areas of the world, prevention efforts are a critical public health measure. In the last decade, prevention efforts have incorporated innovations such as better insecticides and longer-lasting bed nets, helping prevent hundreds of millions of cases of malaria and saving millions of lives. Although mortality rates have fallen, more than 200 million new malaria cases and almost half a million malaria deaths (mostly in children under 5 years old) still occur each year. Such statistics testify to the limitations in current mosquito prevention methods, as do the illnesses and deaths caused by other mosquito-borne infectious diseases, which include Zika, dengue, yellow fever, and chikungunya.
Malaria and other mosquito-borne infectious diseases could be eradicated by gene drive technologies, which are used to modify or eliminate selected species, including disease vectors. Recently, gene drives have been accelerated by CRISPR, which creates excitement and anxiety in every field it touches. In the gene drive field, which is no exception, the power of CRISPR is being wielded with an unprecendented degree of precision.
From zero to extinction
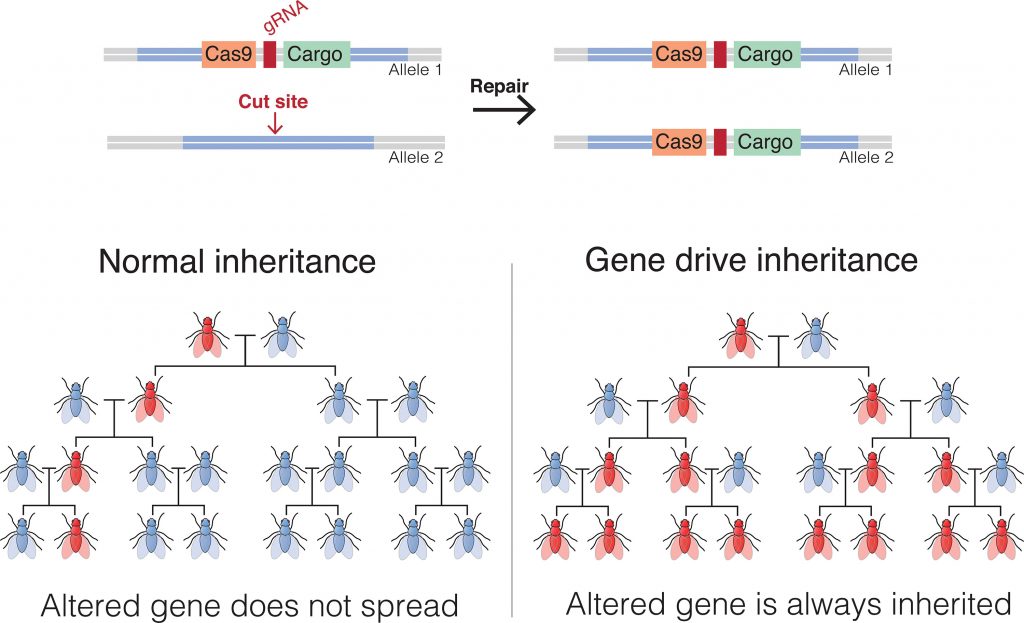
A gene drive is designed to change the odds of inheriting a particular DNA sequence. In doing so, it could alter the presence of a trait in a wild population. When a genetically altered mosquito mates with a wild-type mosquito, 50% of offspring will normally carry the altered gene. A gene drive skews this pattern of inheritance by ensuring that the altered gene will be inherited at a much higher frequency, resulting in a rapid spread throughout a wild population, even if an evolutionary disadvantage is conferred, (see illustration on page 22).
Unlike earlier gene drives, CRISPR-based gene drives show almost complete transmission. A CRISPR-based gene drive carries an altered gene, the Cas9 enzyme, and several guide RNAs. Not only is the altered gene transmitted, but the CRISPR-based gene drive machinery is inherited along with it.
Like many fields that have been touched by the power of CRISPR, gene drive technology is moving so quickly that it threatens to outpace the development of necessary (or at least prudent) checks. For example, there are concerns that CRISPR-based gene drive may have adverse ecological impacts. The possibility that it could eliminate or alter an entire species, even the deadliest animal on the planet, has led some gene drive biologists to turn their attention to developing brakes for this technology.
Some control measures could be built into a CRISPR-based gene drive from the beginning, whereas others could be put into place if needed. “There is no reason not to have countermeasures” insists Amit Choudhary, PhD, assistant professor of medicine, Harvard Medical School, “just in case.”
Gene drive’s many makes and models
The importance of having a countermeasure in place when releasing a gene drive into the environment “depends on the type of gene drive system that you’re talking about,” says Omar Akbari, PhD, assistant professor of cell and developmental biology at the University of California, San Diego. One of the big misconceptions, notes Akbari, is that when people hear the word, gene drive, they think of a system that will spread from a single individual into the entire population across the entire world. He frequently hears words like “self-sustaining,” “invasive,” and “global” used to describe gene drives. But according to Akbari, these words may not apply to gene drives, which come in many different varieties.

Some gene drives are known as “daisy chains” because they possess, like a real daisy chain, many links. The daisy chain, as described by Kevin Esvelt, PhD, an assistant professor of the MIT Media Lab, is like multistage rocket. Each element in the daisy chain is a genetic booster that propels the payload. As the elements are lost, the payload slows down. As a result, the gene drive is both temporary and confined to the local area.
Different ways of building control measures into gene drives are being explored by researchers. Valentino Gantz, PhD, assistant researcher in cell and developmental biology at the University of California, San Diego, tells GEN that it is not really a question of which one is better. Rather, different systems have different characteristics, and one system might be preferable over another depending on what you’re doing.

Gantz’s lab likes to understand the fine details of gene drives and the implications of subtle tweaks. It develops novel ways to split gene drives, primarily in the fruit fly Drosophila melanogaster, experimenting with the guide RNAs and the Cas9 to, in Gantz’s words, “combinatorially optimize the elements.” One split system has a static source of Cas9 and is called a “CopyCat.” Another has a transcomplementing gene drive arrangement that distributes the Cas9 and the guide RNAs to different locations. When separated, these elements are inherited as Mendelian transgenes; but when combined they are capable of being copied and propagated exponentially into a population. Gantz tells GEN that recent results have suggested that the transcomplementing gene drive may have advantages over a full gene drive in the environment—results that he did not expect.
Another method is to make the gene drive conditional on some property, such as needing to reach a threshold to self-propagate. For example, given a threshold of 50%, a number of mosquitoes above 50% of the population size would have to be released—which is a lot. If the population of gene drive–carrying mosquitoes dips below the threshold, the gene drive falls out.
“I don’t see any benefit to using a global drive system over a self-limiting or thresholded system on an island population,” asserts Akbari. “It might be a bit more work, with more releases required.” But Akbari notes that for a field trial, all of the same ecology and the population dynamics could be understood from any of these gene drives. And if everything goes well, then the introduction of gene drives to mainland Africa might be a consideration.
For these drive systems, Akbari notes, you wouldn’t need an extra countermeasure because the countermeasures are built in. “There is a lot of work to be done and still a lot of innovation to be made,” he points out. These are not just “release and walk away” systems. And although these controlled gene drives may require more work, they are the safer option.
Zanzare
Malaria was eliminated in Sardinia after World War II, thanks to an experimental project that imposed the ecological equivalent of a scorched-earth policy. The project, which was initiated in 1944 and funded by the Italian government and the Rockefeller Foundation, didn’t fight malaria directly. Instead, it sought to eradicate all the native mosquitoes (zanzare)—something that draws a chuckle from Andrea Crisanti, MD, PhD, professor of molecular parasitology, Imperial College London. How ironic, he notes, that something that would seem risky today was, back then, the primary goal of an expensive and laborious project. By blanketing the island with the insecticide DDT, three of four of the island’s mosquito species were eliminated. If the project’s history offers any guidance, Crisanti points out, it may be that vector eradiation isn’t necessarily disastrous. In Sardinia, the disappearance of mosquitoes has not incurred any ecological downsides. (He adds that no one misses them.)
Over 15 years ago, Crisanti led a team that modified the genome of the Anopheles mosquito for the first time. Two years later, the mosquito’s full genome sequence was made publicly available. Crisanti has a stance on the elimination of mosquitoes that comes across as clearly as his Italian accent. “We should not deploy any gene drive solution in the field if we believe that we might need a neutralizing technology,” he tells GEN. He continues that while neutralizing technology “may provide comfort to some,” he “firmly believes” that “if we propose a gene drive technology solution for eliminating mosquitoes, it is because we believe that it won’t pose any harm to anyone.” Therefore, we should not make the decision based on whether a capable neutralizing technology is available. Why? It could, he suggests, lower the threshold for making the decision. People may be willing to take a higher risk because that technology is there, like a safety blanket.

Crisanti asserts that we should not bring gene drives to the field if we have any doubts about safety and effectiveness. “Authorization should be given because we have provided the confidence that the gene drive will work and will not pose any threat to the environment, ecology, and health of animals and people,” he says. “Only if we are absolutely convinced that the gene drive technology will work and is safe, in the absence of a neutralizing technology, will we proceed in its implementation.”
That said, the Crisanti lab is currently working on developing new types of neutralizing technology. When asked why, he notes that they work on them because “it is difficult to predict what other people may do.”
Chemical control—the dose makes the poison
Choudhary grew up surrounded by mosquitoes in a “slum-like” environment in India. There, he says, “you don’t need to go after the mosquitoes—they come to your home.” But he did not contract vector-borne diseases growing up, a fact that he attributes, in part, to a “Good Knight.” The Good Knight, put out routinely in Indian homes in the evening, is a rudimentary small-molecule-based device that vaporizes pyrethrins—organic compounds that are similar to those produced by the Chrysanthemum and that have insect-repellent properties. Choudhary wants to leverage this platform that controls mosquitoes by distributing small molecules in the air by building small molecule dependence into gene drive control.
His lab has engineered Cas9 to bring in these dependencies so that a gene drive that is in an off state could be switched on when the mosquitos eat or inhale the small molecule. The extent to which they take the molecule will govern the degree to which the gene drive is switched on and, in turn, the degree of inheritance. Choudhary hopes to fine-tune the population using the analog system, dialing the amount of inheritance to obtain intermediate values. He currently collaborates with multiple labs in the gene drive field, including the Gantz and Akbari labs, to incorporate small-molecule control into gene drive systems.
“You don’t need a helicopter to douse the mosquitoes,” notes Choudhary. “You just need something that already exists in almost every Indian home to bring in small-molecule control, activation or inhibition, of gene drives.” Choudhary notes that this is a localization strategy, but not just for India. “Anywhere something is heated, for example, where dinner is cooked, these molecules could be vaporized,” he remarks. He hopes to develop this platform for any resource-scarce setting.
As for the requirement of neutralizing technologies, Choudhary maintains that control is at the heart of any powerful technology. He adds that the controls should be incorporated in tandem because chaos ensues when controls are built in after the fact. He says that a gene drive without controls “doesn’t feel right” and that the control offered by chemicals is fast and cheap and compatible with mass production. Nonetheless, taking a chemist’s perspective, and speaking as an “outlier” among geneticists, Choudhary emphasizes the challenges in the gene drive world as opposed to those in the chemical world, noting that the gene drive space is “a lot more challenging.”

Hands on 10 and 2
Some people are uncomfortable with the idea of altering wild populations and worried about the unknown dangers of meddling with the current ecosystem. Addressing this point, Gantz points to the relatively unfamiliar problem of invasive species, noting that almost all oceanic islands have invasive mice or rats that are “not supposed to be there” and “create huge problems in the native ecosystem.” There are already major efforts underway to control these invasive animals that have been introduced, ironically, by human activity. So, notes Gantz, a gene drive could be considered “a way to remedy all of this” and to “put things back the way they were before.”

Gantz admits that he is “on the very optimistic side” of the field. Indeed, his worry is the opposite of the worry that is held by most others. His concern is that nothing will happen because the technology will not work as well as its developers had hoped. This is what drives his lab’s focus on optimizing gene drives and building tools that will be useful.
In the film, Human Nature, a documentary about CRISPR and its implications, Ian Hodder, PhD, professor in the department of anthropology at Stanford University, comments that “humans are very good at inventing things, but they are very, very bad at working out what the implications are.” Although that is likely true in most fields, the scientists at the helm of the gene drive field are hyper aware of the power held in the tools they are building, and they are appropriately cautious. Indeed, Crisanti adds that people should know that “gene drive technology could not be in safer hands at the moment—the hands of highly responsible scientists in regulated institutions.” He adds that “a moratorium would give an advantage to those that are not accountable.”


