July 1, 2009 (Vol. 29, No. 13)
New Transfection Systems Tackle Delivery Issues and Off-Target Effects
Will RNAi-based therapeutics provide the next class of blockbuster drugs? If pharma’s acquisition spree of companies with relevant technologies proves predictive, then all bets are on. In 2001, siRNA duplexes were hailed as new tools for studying gene function in mammalian cells, and it was predicted that they eventually would be used as gene-specific therapeutics as well. The subsequent growth spurt in the use of RNAi as a research tool and potential drug target has borne out that prediction. Advancement toward the clinic, however, depends on overcoming some key hurdles, including drug delivery and off-target effects.
Technical hurdles notwithstanding, discovery of the RNAi gene-silencing process as a universal control mechanism in eukaryotes has prompted a major wave of consolidation as pharma and other companies devour the key enabling technologies, and continue to develop new ones.
RNAi’s potential as a drug target has attracted huge investments by pharmaceutical companies, as exemplified by Roche’s 2007 announcement that it had committed “over a billion dollars” to a deal with Alnylam Pharma. A year later, Roche acquired Mirus Bio for $125 million to expand its stable of technologies to enhance delivery and potential clinical use of siRNA.
AstraZeneca followed suit with a three-year R&D collaboration with Silence Therapeutics calling for that company to ultimately receive about $400 million for up to five siRNA-based drugs aimed at specific targets provided by AstraZeneca.
Key to the rapid growth of RNAi applications have been new tools and technologies that simplify its use, decrease its cost, and increase its accessibility in a wide variety of research settings. Strategies employed to activate the RNAi pathway and specifically silence genes include introduction of synthetic siRNA into cells or DNA vectors expressing short hairpin RNAs (shRNAs) or miRNAs.
RNA interference can also be achieved in mammalian cells using plasmids containing shRNA. As the field has advanced, so has the realization that multiple factors influence the success or failure of RNAi studies; chief among these are transfection method, transfection conditions, cell type, whether the cells are adherent and confluent or nonconfluent, or in suspension culture, the quality and quantity of siRNA, reagent toxicity, and in the case of in vivo and clinical studies, siRNA delivery.
Several companies are developing methods and reagents to address some of the major challenges in using RNAi-related technologies and potentially enable their use as therapeutic agents. Most view poor transfection efficiencies arising from a variety of conditions as a significant source of compromise to experimental results.
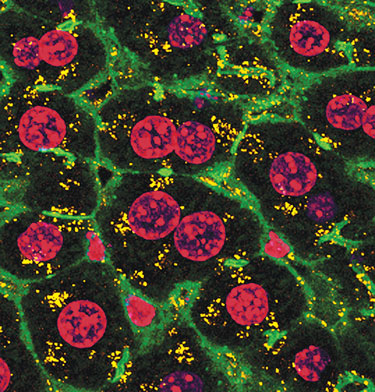
Confocal micrograph of a mouse liver section shortly after simple intravenous injection of hepatocyte-targeted Dynamic PolyConjugate siRNA vehicles.
(So Wong, Roche)
Electroporation in a Pipette Tip
Christofer Cunning, Ph.D., senior marketing manager at Life Technologies, says that reproducibility, toxicity concerns, and the ability to successfully transfect primary cells and difficult-to-transfect cells, including immune cells and stem cells, remain challenging in siRNA experiments. “In general, people have used vector approaches with viral delivery systems for difficult transfections,” he says. “Use of viral vectors, however, remains time-consuming, requires cloning steps, is relatively expensive initially, and may require significant safety precautions.” In addition, while electroporation has been widely applied, “it requires strict adherence to already set protocols and a lot of siRNA.”
To address these issues, Invitrogen launched its Neon™ transfection system earlier this year. In contrast to other systems, Neon allows transfection to take place in a pipette tip, using as few as 2×104 cells. “This technology is amenable to changes in protocols that can be defined by the user,” Dr. Cunning asserts.
Unlike standard cuvette-based electroporation chambers, the Neon system uses a biologically compatible pipette tip chamber that generates a more uniform electric field. The company says this design allows better maintenance of physiological conditions resulting in high cell survival compared to conventional electroporation. A universal reagent kit allows use with all cell types.
Invitrogen recently launched Invivofectamine™ for in vivo siRNA transfection. The company claims that in contrast to other marketed products, Invivofectamine can be injected in small volumes (microliters as opposed to milliliters) and without high pressure, thereby minimizing the potential of inconsistent results and gross harm to the subject animal.
This reagent also reportedly provides stability to siRNA so that it remains intact and ready to perform the selected knockdown. Invitrogen has demonstrated successful delivery of intact siRNA molecules via direct injection into established tumors, tail vein injection to the liver, kidney, lungs, spleen, and pancreas, as well as effective and specific knockdown in these tissues.
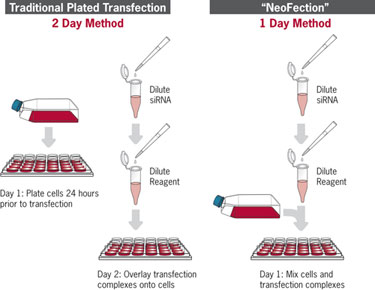
Standard preplated transfection vs. reverse transfection with siPORT™ NeoFX™ transfection agent
(Ambion/Life Technologies)
Dynamic PolyConjugate Transfection
Roche Madison was formed following Roche’s purchase of Mirus Bio in 2008. It has developed Dynamic PolyConjugate (DPC) technology specifically to transfect and deliver siRNA in vivo. David Lewis, Ph.D., program director for RNAi, says the special properties that enable in vivo siRNA delivery include a positively charged membrane-active polymer that can act as a transfection agent once inside a cell.
“We shield, or mask, the positive charge for in vivo use and covalently bind siRNA to the polyconjugate via a disulfide bond,” Dr. Lewis explains. “The polymers are directed to specific locations through targeting ligands, for example, n-acetyl galactosamine, that targets the polymer to liver hepatocytes. Through the targeting ligand, the polymer can engage asialo-glycoprotein receptors and be taken up into liver cells via receptor-mediated endocytosis.”
In the endosome, the polymer is unmasked and becomes cationic, he says, allowing it to act as a transfection agent. The polymer lyses the endosome, “exposing it to the cytoplasm, a reducing environment that reduces the disulfide bond tethering the siRNA to the polymer and frees it to engage with RISC complexes.
“To date, we have been able to silence apolipoprotein B as well as other target genes in rodents and nonhuman primates, and we envision this technology as a potential delivery system for therapeutic siRNAs,” Dr. Lewis adds. “Following apoB siRNA delivery, we have seen a decrease in LDL and serum cholesterol in the animals.” In addition, DPC can be delivered by simple intravenous injection in small volumes and does not require injection in potentially harmful large hydrodynamic volumes.
Nanoparticle siRNA Transfection
Sigma-Aldrich’s N-TER™ nanoparticle siRNA transfection peptide-based system was developed to circumvent a number of drawbacks associated with lipid-based siRNA transfection agents. These include limited ability to transfect a variety of cell lines such as primary, neuronal, differentiated and nondividing cells; toxicity; and relatively longer times required for reagent uptake and subsequent target knockdown to occur.
Designed for use in transient knockdown of eukaryotic gene expression, the N-TER peptide binds siRNAs noncovalently, forming a nanoparticle that can interact directly with lipids on the surface of the plasma membrane, allowing the nanoparticle to cross the cell membrane and deliver the siRNA directly to the cytoplasm.
According to Steven Suchyta, product manager, functional genomics, this siRNA delivery system enters the cell via an as-yet undetermined pathway. The delivered siRNA becomes available to the RNAi interference machinery soon after entry into the cell.
“N-TER/siRNA complexes are generally more stable than a liposome,” Suchyta says, “and we have seen faster uptake and relatively long persistence within the cell.” N-TER is based on an amphipathic peptide that interacts directly with the siRNA to form a nanoparticle, which can efficiently deliver and release the siRNA into the cell.
In pointing out advantages that distinguish peptide-based nanoparticle delivery, Suchyta comments that this reagent exhibits reduced toxicity compared to lipid-based reagents. Particularly, he says, it is effective in extremely difficult-to-transfect cells. Recent experiments with the undifferentiated mouse skeletal myoblast cell line C2C12 could be successfully transfected with slight protocol optimization, for example, by varying cell densities.
Typically N-TER can work over a wide range of confluencies and works well even for cell types that are typically highly confluent such as HUVEC cells, he adds. Sigma Aldrich is now “exploring the potential in vivo use of N-TER,” he reports. “We have seen some promising initial data in mouse models. Allowing scientists to take the next step in their RNAi research using animal models is one of Sigma’s highest priorities, and we will continue to actively pursue technologies that facilitate in vivo RNAi.”
Through its Dharmacon products, Thermo Fisher Scientific offers siRNA delivery “without a transfection reagent,” says Devin Leake, Ph.D., director of research and development for genomics products. Dr. Leake and his colleagues have developed methods for siRNA design and identified chemical-modification patterns to enhance potency, stability, and specificity of siRNA transfection. Dr. Leake says that while lipid reagents were designed to get siRNA into “as many cell types as possible, certain cells including stem cells, immune cells, and primary cells remain challenging.”
According to the company, its Accell siRNA promotes cellular uptake in many cell types without the toxic and inflammatory responses typically associated with conventional lipid transfection agents. Further, it claims, the “passive nature” of these reagents reduces adverse off-target gene knockdown.
Dr. Leake explains that these chemically modified siRNAs enter almost any cell type directly from the culture medium. While transfection with the modified RNA requires no serum, or reduced serum conditions, “with assay optimization, a window can be found that allows transfection to occur, then cells can be returned to their normal serum-containing medium.”
According to Dr. Leake, researchers have reported successful stem cell, T-cell, Jurkat, and cadiomyocyte transfection with Accell siRNAs. The combination of chemical modifications and sequence selection provides silencing tools in these relevant cell types.
Transfection in Reverse
Ambion was acquired by Applied Biosystems in 2006 and is now a division of Life Technologies. The Ambion brand encompasses a range of products for isolation, detection, quantification, amplification, and characterization of RNA, including its siPORT NeoFX® neotransfection agent, a lipid-based formulation that the company claims can be used to efficiently transfect adherent cells as they are subcultured without increased cytotoxicity
According to Ambion, low transfection efficiencies as well as poor cell viability are the most frequent causes of failed gene transfection studies and that use of reverse transfection, or neofection, reduces failure rates.
Michel Cannieux, product manager, Ambion RNA interference technologies, has found that reverse transfection technology addresses the problem of getting reproducible transfection efficiencies from “well to well, and tube to tube.”
Traditional transfection requires that cells be passed 24 hours prior to transfection. “In traditional transfection techniques, on the day of transfection, complexed siRNAs, transfection reagent, and often a transfection aid are added to the wells in a relatively small volume, to the larger volume of culture medium containing the cells. The addition of a small volume to a larger one results in inefficient mixing, particularly in the case of 96- to 384-well plates.”
As a result of poor mixing “some cells receive a larger dose of transfection reagent, leading to localized toxicity and transfection inefficiencies within each well.” By contrast Cannieux says that, in reverse transfection the siRNA and transfection reagent contained in just a few microliters are distributed first into the wells. The cells in a 10–20 larger volume of culture medium are overlaid onto the mixture in the wells while the cells are still in suspension.
Cannieux says that while efficient transfection is crucial for the outcome of any RNAi experiment, with chemically synthesized siRNAs in particular, “you don’t have the luxury of being able to eliminate nontransfected cells as you would using a selection marker on a plasmid vector. High-transfection efficiency and a robust and reliable protocol that works for most cell lines is important and become paramount for siRNA screening programs. Transfection efficiency has to be comparable from plate to plate, for the whole set of siRNAs tested, sometimes several thousands, as in the case of genome-wide screens.”
Patricia F. Dimond, Ph.D. ([email protected]), is a life science consultant.



