December 1, 2011 (Vol. 31, No. 21)
NMR-Based Assay Method Seeks to Improve the Productivity of Biologics R&D and Processing
Among the FDA-approved molecules reaching the market in recent years, new biologics and biosimilars have steadily increased in importance and are expected to continue to do so for at least another five years. Big Pharma companies are currently increasing the biologics component of their portfolios, so that vaccines and protein therapeutics will represent about 20–25% of their income by 2014. Consequently there is a significant drive to access technologies that promote optimal yield as well as ensure the highest possible level of quality and control.
Biologics manufacture can be a very complex process where batch variability or failure cannot be fully understood or easily solved, as there are so many parameters to consider. Biotech companies are therefore starting to examine more rapid and efficient methods to support biologics process development as well as achieve a higher level of control in manufacturing.
To tackle the problem, Spinnovation Biologics introduced Spedia-NMR™. This technology uses nuclear magnetic resonance (NMR) assay methods to rapidly analyze levels of feed components and metabolites within culture media samples prior to, or during, the fermentation process.
With limited sample preparation, high reproducibility, and a linear response over a wide range of concentrations (1 µM to 1 M), NMR offers specific advantages over LC methods. Requiring no systematic calibration and unhindered by matrix or pH effects, NMR offers multiplex analysis with access to structural as well as quantitative data. It is also more amenable to analyzing a wide variety of components that can exist, including polar, nonpolar, volatile, or nonvolatile molecules.
Spedia-NMR is capable of analyzing over 50 components simultaneously, resulting in a high sample capacity of around 100 samples/day. With a typical limit of detection of 1–10 µM, and a limit of quantification of 50–100 µM, the method is suited to the analysis of defined as well as undefined media (e.g., hydrolysate) and delivers rapid detection and identification of unexpected components, such as impurities.
Adopting NMR assay techniques will help both research and upstream processing teams to speed up and improve the development process for biologics, with concomitant commercial implications (lowering costs and accessing the market more quickly).
Besides the obvious market advantages, quality by design (QbD) has recently become much more important with QbD principles being actively promoted by the FDA, and this could also benefit from such a versatile and accurate analysis method.
Adoption of NMR profiling, as part of the process analytical technology used during the setting of critical process parameters, could well facilitate the achievement of QbD standards in biologics development and manufacturing.
Because the NMR-based approach can evaluate over 50 different components at a time, it is highly suited to the evaluation of cell-based production systems for biologics. The sample report details the absolute concentrations of components such as metabolites, contaminants, and feed material, providing an overall snapshot of the cell culture medium at a particular point in time.
This capability provides R&D teams with an efficient way to accurately follow the effects of each component on cell growth and protein production. By following the process over a number of different samples, taken at regular time intervals, a detailed profile can be built up correlating the influence of feed components and metabolites on the progression of cell culture.
Case Study
A client requested the monitoring of as many analytes as possible in a CHO cell culture to support the upstream process development of a therapeutic protein.
Sixteen culture media samples of about 1 mL were collected from a straight-batch over an eight day period. After minimum sample preparation—centrifugation and filtering—the samples were analyzed by Spedia-NMR. The absolute concentration of over 30 components was rapidly derived (matter of minutes/hour) for each time-point of the culture.
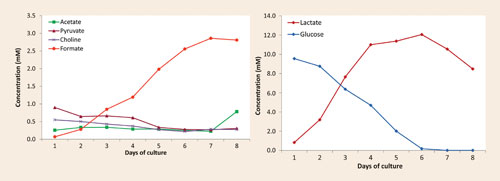
The results, available the day after the reception of the samples, were presented in the form of a concentration profile of various components over the sampling period. They showed a rapid increase in both formate and lactate levels (Figure 1) and a rapid decrease in the level of the amino acid asparagine (Figure 2).
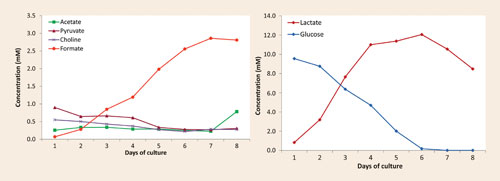
Figure 1. A significant and steady increase in formate level was observed (left) as well as an increasing lactate level and glucose consumption (right) in the same cell culture (straight-batch).
This information highlighted the main production-limiting issue: the rapid degradation of the amino acid was causing the release of ammonia, which was highly toxic to the cells in the culture. The results also indicated that the rapidly increasing and sustained lactate levels needed to be addressed in order to further improve the culture performance (cell density, viability, and protein production).
Following process modification, a similar batch was closely monitored by NMR and compared to a control batch. In this case the client had applied a new technique to help reduce the lactate in mid-term culture, while also providing additional glucose feed material. This led to a rapid improvement of the results with much better lactate control and provided data to validate the new process optimization. The information on amino acid consumption was also used to fine-tune the media conditions for optimal protein production.
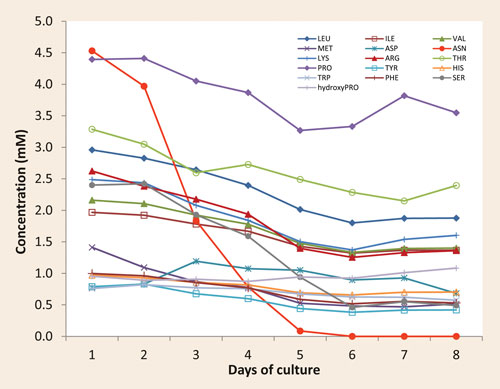
Figure 2. A rapid and dramatic reduction in the level of asparagine during the cell culture process (red-line ASN) was observed, a result of consumption and degradation. An increase in aspartic acid, product of the asparagine degradation, was also seen.
The Future
NMR is now set to become a prominent method in both the industry’s process and product bioanalysis toolbox. The analysis of culture media using Spedia-NMR technology is versatile, rapid, and robust and is particularly suited to examining whole culture media samples (complex matrix) within a few days.
NMR-profiling approaches are typically nontargeted, and it can be advantageous to investigate a process quickly and simply with no need of a starting hypothesis. The data obtained can rapidly guide researchers on how processes may be modified to increase both performance and quality, e.g., in relation to recombinant protein production.
The combination of automation and low-detection limits promotes fast-throughput NMR analysis capability, greatly reducing the costs of analysis per sample. These improvements can only increase the usefulness of NMR assay techniques and drive the continued adoption of NMR in the characterization of cell culture media.
Frederic Girard ([email protected]) is CEO at Spinnovation Biologics.