March 15, 2013 (Vol. 33, No. 6)
Protein expression systems are notoriously difficult to work with, but a bevy of researchers have found solutions that have facilitated their protein therapeutic work.
At ISBiotech’s meeting held earlier this month, speakers shared their success stories.
Penny L. Post, Ph.D., vp, regulatory affairs, Protein Sciences, and her colleagues have designed a process that involves culturing insect cells in a bioreactor, infecting the cells with a baculovirus vector engineered to contain the gene of interest, incubating the infection for the appropriate amount of time, and purifying and formulating the expressed product.
“Using this system, we recently received FDA approval for the first recombinant trivalent hemagglutinin (rHA) influenza vaccine,” shared Dr. Post. “The vaccine is cloned from FDA-selected vaccine strains. One important feature of the system is that we have eliminated the need to handle live influenza viruses. Since the manufacturing expression system does not require eggs or actual influenza viruses, no adaptation of the influenza virus is required and an exact genetic match to the viral hemagglutinin can be produced.
“This is advantageous for pandemic preparedness, as an inherently safe system for the production of vaccines for pandemic influenza and for enhancing production speed and vaccine availability.”
Dr. Post noted that the baculovirus vector insertion is being changed, and not the cell line. Therefore, a single qualified cell line and a single master baculovirus bank can be used for all products, allowing for rapid start-up of new products and faster regulatory approval.
“The system has the ability to express high levels of protein, and multiple genes may be co-expressed. The technology may be used for the production of a broad range of protein-based vaccines for both human and veterinary use,” she added.
The production bioreactor is loaded with cells from the working cell bank and then infected with the working baculovirus bank containing the gene(s) to be expressed. After 48 to 72 hours, the bioreactor is harvested, and product is purified with a series of chromatography and filtration steps, formulated into the final buffer, and filtered through a 0.2 µm filter. The product is then compounded, undergoes final filtration, and is filled into vials.
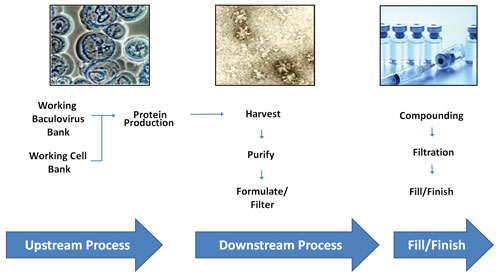
Baculovirus expression vector system process improvement cycle at Protein Sciences
Virus-Like Particles
In the production of recombinant virus-like particles (VLPs), cell culture methods that utilize the baculovirus vector expression system are commonplace. VLPs are highly immunogenic. Morphologically and antigenically, they resemble real viruses, yet are noninfectious as they do not contain a viral genome.
VLPs provide an alternative to the egg-dependent approach for the production of influenza vaccines. With current VLPs, influenza proteins are made and assembled into VLPs as individual vaccines for each strain of virus. These VLPs are then blended to produce multivalent influenza vaccine formulations.
Peter Pushko, Ph.D., president and CSO, Medigen, advocates use of an alternative VLP design for influenza vaccines. “Similar to current licensed trivalent vaccines, VLPs with one subtype of hemagglutinin (HA) represents strain-specific preparations that are generated separately and subsequently combined,” he explained.
“Our recombinant vaccine design results in the expression of three HA subtypes within the VLP envelope. By incorporating more than one subtype of HA into the VLP envelope, we have provided multisubtype VLPs that induce protective immune responses against multiple strains of influenza.
“This VLP concept could be applicable for the preparation of vaccines for biodefense purposes, such as vaccines against yellow fever and Lassa fever viruses,” Dr. Pushko noted.
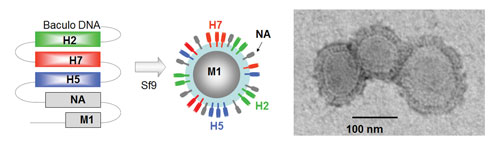
Influenza genes including H2, H7, and H5 hemagglutinin genes are expressed in eukaryotic Sf9 cells by using baculovirus expression vector system (BEVS), as shown on the left panel. Influenza proteins self-assemble into multisubtype VLPs (in the middle), which protect from multiple pandemic influenza viruses. Electron microscopy (on the right) shows purified VLPs of 100 nm in diameter. [Medigen]
BacMam Technology
Large-scale transient gene expression systems provide the ability for rapid production of recombinant proteins by transfection of the polyethyleneimine (PEI) plasmid in HEK-293 and CHO cell lines. Plasmid-PEI complexes are readily taken up by HEK-293 cells and, to a lesser extent, into CHO cells.
These systems are not without their challenges, which include the need to generate large quantities of purified, low-endotoxin plasmid, the limited number of commercially available serum-free media formulations that support PEI-mediated transfections, and the overall cost of commercially prepared PEI reagent.
The BacMam technology provides an alternative system to large-scale transient gene expression in mammalian cells. In the BacMam technology, baculovirus particles carry mammalian promoters in place of the polyhedrin promoter. These BacMam viruses transduce a wide variety of mammalian cell lines and numerous cell-based high-throughput lines, thus enabling the utilization of BacMam transduced cell lines instead of stable cells.
Christopher W. Kemp, Ph.D., president, Kempbio, is using BacMam transduction to generate recombinant proteins in large-scale transient transfections.
“Initial experiments conducted last year revealed that protein expression levels in HEK-293 cells were equivalent between the PEI and the BacMam methods,” he explained. “The ability to rapidly produce protein complexes on a short time line is a hallmark of the BacMam technology. Starting from variable region gene sequences, we have been able to produce and purify 100 mg of purified rIgG in eight weeks using the BacMam expression system.
“The protein had an endotoxin level of 0.7 EU/mg without utilizing endotoxin removal techniques. The aggregation level of the rIgG was
Dr. Kemp noted that a cost analysis comparing BacMam and PEI-based methods revealed that the cost of production was 25% lower using the BacMam method.
“The cost reduction was due mainly to the cost differential between baculovirus amplification and plasmid amplification/purification and the need to remove endotoxin from the final product,” he added.

Characteristics of a murine rIgG produced using BacMam transduction of CHO-S cells: The monomeric nature of the purified rIgG is demonstrated by the absence of a leading shoulder in the size-exclusion chromatograph. The rIgG was expressed using a defined protein-free medium and purified using Protein G affinity resin. The Coomassie-stained SDS PAGE gel illustrates the level of purity of the rIgG and the uniform nature of the heavy and light chains. [Kempbio]
Drug Discovery
James M. Groarke, Ph.D., partner at GroarkevonStein, is involved in supporting the establishment of the baculovirus expression platform for the production of recombinant proteins.
“Our focus is drug discovery,” he said. “In order for the baculovirus expression vector system to be used to its full potential, certain critical features must be attained. These features include the ability to utilize healthy, low-passage cell lines, high-quality media, correct multiplicity of infection, and the appropriate harvest time post-infection.
“To attain these critical features, certain challenges associated with the baculovirus expression system in relation to protein production must be dealt with. These challenges include adherence to internal quality control standards, optimization of expression conditions with respect to the multiplicity of infection, and the time course of the infection and the expression of multiprotein complexes.”
Optimization
Given the challenges and the demands for recombinant protein production, the need for optimization of the baculovirus expression vector system is apparent.
Fuad T. Haddadin, Ph.D., senior scientist at Boehringer Ingelheim Vetmedica, works on the process development and improvement of biological vaccines, with a focus on the baculovirus expression platform, using the scale-down approach.
“Time-to-clinic (TTC) is considered critical during early development of new processes into manufacturing,” Dr. Haddadin explained. “Yet, it is important to maintain a balance between process quality and TTC as project-management practice is framed to maximize the outcome of time, cost, and quality. This constantly creates a challenge for bioprocess engineers to improve process productivity and consistency during the post-clinical phase. For large-scale process optimization, the scale-down approach is often employed to efficiently generate data on a small scale, and effectively apply suggested improvements at the large-scale.”
“Therefore, it is imperative to ensure that scale-down conditions are representative of the real large-scale bioprocess,” explained Dr. Haddadin. “This can be accomplished by understanding the interactions of various engineering, biological, and chemical factors that affect bioprocess yield and consistency. The effective implementation of a systematic scale-down approach can lead to significant improvements in process yield and consistency in manufacturing.”



