November 15, 2015 (Vol. 35, No. 20)
Serious Capacity Problems for CMOs Seem to Be a Thing of the Past
A contract manufacturing organization (CMO) with excess production capacity is like a restaurant with unfilled tables—it points to excess overhead, low profitability, and poor planning. Yet full capacity can sometimes be worse in the long run through lost business and permanently unhappy clients. So CMOs balance their capacity to ensure future growth and long term demand. And when properly managed, moderate capacity constraints can be a healthy indicator of efficient operations.
Our 2015 Annual Biopharmaceutical Manufacturing Survey, however, shows that CMOs in U.S. and Europe are hitting limits of what may be considered a healthy level of excess (flex) capacity. This is particularly the case for mammalian cell culture—the dominant platform. In our study, CMOs this year estimated they are operating at an average 82% of their total capacity. This is a hefty increase from last year’s average of 58% capacity utilization. The last time we saw levels in this range were 10 years ago during what was often referred to as an industry-wide capacity crunch.
CMOs’ success is partly the result of industry maturation and experience in scheduling, managing the pipeline products, and clients’ expectations. In addition, the biopharma industry continues to see a robust rate of new product approvals. Overall, outsourcing of biomanufacturing has been on the rise and CMOs have honed their ability to keep up with demand. Thus, CMO constraints are not necessarily an unhealthy indicator. And results from this year’s study show CMOs are also very optimistic regarding management of their capacity in the future, as they invest in capacity improvements.
Our global study of 237 biopharmaceutical manufacturers and CMOs shows, for example that while only around 10% of biotherapeutics developers will experience significant constraints, over one-third of CMOs are experiencing at least “significant” production capacity constraints. And while these numbers are up substantially from last year, they’re still anecdotally being pegged at reasonably healthy capacity levels.
So the question should be whether the current CMOs capacity pinch is necessarily bad news, or is it an indication of efficient project management, that is to be followed by a period of expansion.

CMOs balance their capacity to ensure future growth and long-term demand. [iStock/gong hangxu]
CMOs Expanding Facilities
Our expectation is that CMOs are managing their growth effectively. In fact, many are very positive about their ability to handle future capacity constraints. For example, spending on new facility construction is going to occur among nearly 70% of CMOs. And the overall budget increases for facilities are outpacing spending hikes in other areas, including chronic problem areas like staff hiring, process development, and new technology investment.
CMOs this year estimate they will expanding their mammalian cell culture capacity by 77% over the next 5 years, double the projections they forecast just last year. These forecast expansions are evidence that CMOs expect business to continue booming. And market factors like the introduction of biosimilars on the U.S. market are likely to favor CMOs, as well.
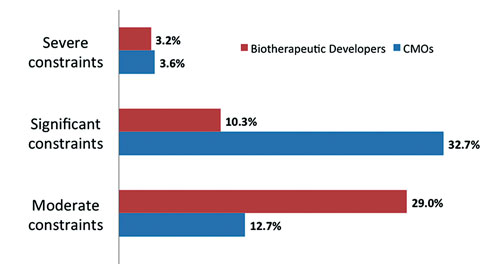
A comparison of the capacity constraints for biotherapeutic developers and those for contract manufacturing organizations (CMOs). [12th Annual Report and Survey of Biopharmaceutical Manufacturing, April 2015]
How CMOs Are Avoiding Constraints
CMOs identified factors creating production capacity constraints at their facility over the next five years. General facility constraints topped the list; however, the capacity of downstream purification equipment was the second-most likely factor. Improvements in purification technology are emerging that will address these problems, and suppliers are investing heavily into novel purification approaches including:
- Moving bed technologies
- Membrane separation technologies
- Better UF-membranes
- Better resins, including faster flow and greater binding capacity
- In addition, more single-use equipment for purification is coming online
CMOs are often first to evaluate and implement new bioprocessing technologies. So although downstream capacity is an issue for CMOs, they are being pessimistic about investment until truly novel solutions arrive that will dramatically improve their downstream processing. Beyond facility and downstream equipment constraints, CMOs also see staffing problems as contributing to future capacity constraints, with many pointing to an inability to retain experienced technical and production staff (noted by 42% of CMOs).
CMOs and biomanufacturers are in general agreement about the important areas to address in fixing capacity problems. New product development areas, especially for CMOs, include disposable purification products, chromatography products, and other technologies that create more efficient downstream purification. CMOs are also concerned about staffing issues that can contribute to chronic problems.

Certain factors are expected to create capacity constraints for both biotherapeutic developers and CMOs over the next five years, though to differing degrees. [12th Annual Report and Survey of Biopharmaceutical Manufacturing, April 2015]
Eric S. Langer ([email protected]) is president and managing partner at BioPlan Associates. He is editor of numerous studies, including “Biopharmaceutical Technology in China,” “Advances in Large-scale Biopharmaceutical Manufacturing”, and many other industry reports.



