
Exciting times lie ahead for companies that provide animal models. Many of these companies are finding ways to help drug developers accomplish increasingly ambitious tasks, such as shifting from small molecules to large biologics, evaluating therapeutic approaches to complex diseases, and taking account of genetic diversity, microbial composition, and other subtleties.
Animal model providers are taking advantage of next-generation sequencing technology, genome engineering tools, and advanced computational approaches such as big data analytics and machine learning. At the same time, animal model providers are finding new ways to ensure that in vitro testing and in vivo testing complement each other. For example, organoid systems are being used to guide the engineering and improve the selection and use of animal models.
In this article, five leading animal model providers describe the current state of the industry, discuss emerging trends, and highlight new applications.
Humanization and customization
Since 1997, research projects enabled by genetically modified models has doubled. This increase reflects a durable trend, says Steve Festin, PhD, director of scientific and commercial development at Charles River Laboratories. He suggests that genetically modified models will continue to be in demand because they can help researchers identify and validate therapeutic targets. In many studies of complex human diseases, models are needed that include more than one engineered genetic modification.
Since 2012, CRISPR-based genome editing has been combined with commercial-quality breeding to improve multitarget engineering, giving it the speed and accuracy to expedite the production of transgenic and knockout models, including models that mimic human immune responses or are immunodeficient. Other models are available that harbor human-like microbiomes.
Animal models from Charles River are available to facilitate studies in various therapeutic areas from metabolic disease to cancer. The company also supplies microbiome models and custom rare disease models focused on supporting personalized therapy drug trials. “The growth of personalized medicine is driving the demand for humanized mice models in both developing and developed countries,” Festin points out. “The trend toward more human-like animal models and primary tissue/cells will continue, as will the need for highly customized models to assess target validation, efficacy, and safety in cellular therapies.”
Growing complexity
“Traditional inbred and outbred animal models are more adaptable to different therapeutic interventions in a small-molecule setting, allowing testing of a broader range of molecules with a relative expectation of the outcome,” says Michael Seiler, PhD, vice president of commercial products at Taconic Biosciences. “The switch to high-complexity therapeutics, such as monoclonal antibodies, requires different critical components.”
Humanized immune system mice can be genetically engineered to express supportive growth factors that complement the introduction of human hematopoietic stem cells into immunodeficient mice to expand development of immune cell sublineages. Model decisions depend on the immunologic paradigm being tested. Taconic offers a range of transgenic immunodeficient strains.
“A game-changing element is the influence of the microbiome on host systems and the drug-testing paradigm,” Seiler notes. “In general, model suppliers have relied on testing and exclusion to control the microbial components of research animals. Now, with the disruptive, more detailed appreciation of the microbiome and its effect on experimental outcomes, we think about inclusion.”
Animal models from Taconic are available that can help drug developers understand the microbiome’s contribution to experimental variability. For example, the TruBIOME portfolio starts with germ-free mice, like a clean slate. Loss of genetic function can be added through genetic manipulation of a gene or pathway, then combined with a predefined microbiome association.
Existing models are also being reevaluated for new purposes. For example, one of Taconic’s transgenic models, the Jh mouse, has a targeted deletion of the Ig locus, the pathway that leads to B-cell development and the production of antibodies. The deletion eliminates the possibility of antidrug antibody development and permits longer-term evaluation of bioavailability and efficacy. The recently introduced diet-induced nonalcoholic steatohepatitis model is another example, where the standard C57 black 6 strain gains a more relevant disease phenotype based on special diet conditioning.
“Our end game is to build the most potent, information-packed models that reflect aspects of human disease to facilitate drug discovery,” Seiler declares. “The clinical benefit of immuno-oncology indicates that researchers are going to begin moving upstream to focus on interception. We need to understand these trends and how our models interact with other evolving technologies, and we need to adapt accordingly.”
Increasing need for diversity
Although animal models have enriched our understanding of biology, they have had less success driving clinical translation. According to Michael Ellis, PhD, associate director of product management and business analytics at the Jackson Laboratory, three key trends influence the continued relevancy of animal research models in future biomedical research and drug discovery.

Source: Jackson Laboratory
The first trend is the increasing focus on the use of advanced tools to create complex constructs to model disease or an underlying biological mechanism. With these models, there is better recognition of the important role genetic background plays, especially in studies of complex, multigene mechanisms. According to Ellis, “Building a complex genetic model on the wrong genetic background can lead to a nonfunctional model or, even worse, one that is functional but with impacted translational relevance.”
The second trend is better recognition of the importance of genetic diversity. “Research based on a single inbred mouse strain is analogous to research based on a single human patient,” Ellis explains. “[There is] very limited potential for broader understanding or inference.” This is especially important when studying complex, multigene diseases—which are very likely to be present and to show large variability between individuals—or when conducting drug safety screens where population dynamics are a key measure of overall safety.
To address this need, the scientific community has developed many genetic diversity resources, including the Collaborative Cross and Diversity Outbred mouse models. To ease the shift toward genetic diversity, the Jackson Laboratory makes these models and associated tools readily available.
The third trend is the community’s increased focus on the use of humanized models—both genetic and engrafted—to better model human biological mechanisms not only in oncology, but also in other inflammation-driven disease processes from metabolic to autoimmune to cardiovascular disease. The Jackson Laboratory’s comprehensive portfolio of human immune-engrafted models—based on the next-generation sequencing mouse model—includes models for whichever immune compartment is of interest.
Addressing attrition rates
“The high attrition rate of new anticancer agents due to lack of efficacy and toxicity is a pressing issue,” notes Henry Li, PhD, CSO of Crown Bioscience. “Developers need models that more faithfully recapitulate the genomic and immunological heterogeneity of the patient population, as well as models that reflect the changing landscape of resistance and relapse.”
CrownBio’s models may be used to validate targets, elucidate mechanisms of action, and evaluate efficacy and toxicity. For example, the company has developed HuGEMM, a platform for immunotherapy evaluation. “Our HuGEMM immunocompetent chimeric mouse models are engineered to express humanized drug targets, such as genes encoding for immune checkpoint proteins, which can be directly used to evaluate therapeutics,” Li points out. Another one of CrownBio’s platforms is MuPrime, which consists of tumor homograft models that have been engineered to incorporate human disease pathways. MuPrime models featuring oncogenic drivers, for example, can be used to evaluate responses to targeted therapies and/or combination immunotherapies.
In addition, more early-stage complementary in vitro tools, such as organoids select miniature, simplified versions of human organs union are being used to inform decisions in animal models. CrownBio is developing its organoid platform for higher-throughput screening using patient-relevant material, in addition to expanding its patient-derived xenograft select PDX) collection through links with clinical centers. These collaborations aim to generate models that can advance resistance and relapse studies, including those that rely on imaging technology.
“The need for better patient stratification and biomarker identification is driving more analysis of animal models ex vivo and in silico, enabled by big data analysis approaches using the vast collections of data generated from animal models, so we are extending our biomarkers and bioinformatics capabilities,” Li explains. “As we understand more about cancer and unravel new targets and mechanisms, we need to innovate and adapt our in vitro and in vivo models appropriately.
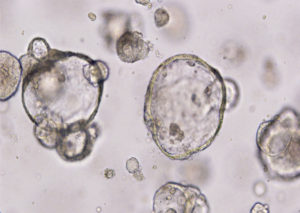
Alternatives to animal models include organoids, which are self-organized three-dimensional tissue cultures, or “organs in a dish.” Organoids derived from stem cells are available from Crown Bioscience. The company asserts that these organoids faithfully recapitulate original patient tissue and are extensively characterized by pathology, genomics, and sensitivity to known and
“The mechanisms of resistance and relapse in patients who have received today’s targeted and combination immuno-oncology therapeutics will be clarified. Technological advancements, such as improved in vitro screening, and a focus on ex vivo analysis will continue to refine animal models and their applications.”
Evolving into collaborators
The scientific community is still far from being able to fully understand diseases based on cell-level or in vitro studies. Consequently, it has been turning to humanized animal models, observes Biocytogen. According to the company, demand for these models has been increasing across all sectors. Important applications include target-humanization and human immune-system reconstitution in immunodeficient mice.
“The advancement of gene editing technology, improved understanding of translational science, and accumulated sequence data are driving the demand,” says Qingcong Lin, PhD, senior vice president of Biocytogen and CEO of Biocytogen Boston. “With better access to patient cancer cells and the accumulation of tumor sequence data, animal model providers are able to produce better PDX models for cell and immunotherapy.”
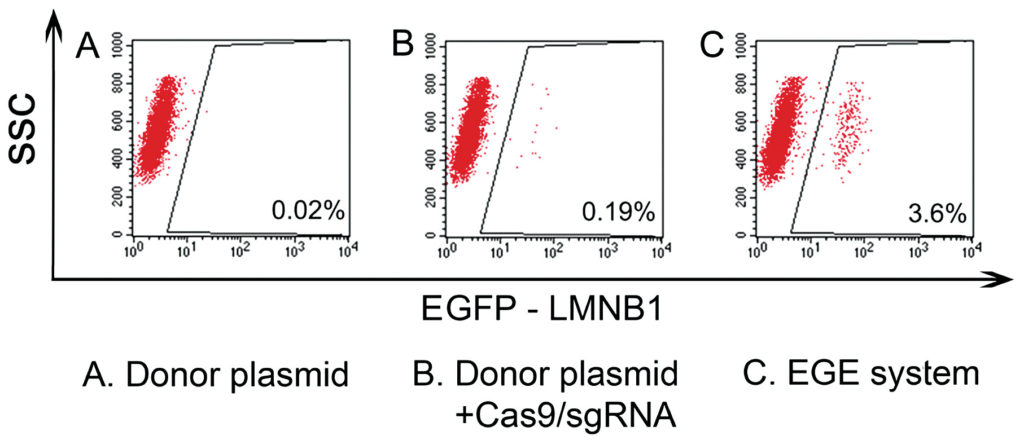
Biocytogen provides a selection of mouse models that includes double- and triple-humanized immune checkpoint mice. The company’s Extreme Genome Editing select EGE) technology can increase the efficiency of the conventional CRISPR-Cas9 technique by up to 20-fold. In the next two years, the company plans to generate humanized mice models for over 1400 disease targets.
“We believe that humanized models will become increasingly important tools for in vivo preclinical efficacy studies in applications ranging from immuno-oncology to more complicated models, such as autoimmune and neurological diseases,” Lin continues. “We also see animal model providers evolving into collaborators and playing bigger roles in the drug discovery process.”
The company has successfully generated the fully human antibody mouse, RenMab™, which upon immunization generates a complete repertoire of fully human variable regions as shown by sequencing data of the antibody gene and CDR3 analysis of the antibodies produced. Additionally, common light-chain- and heavy-chain-only versions of RenMab will be available soon for the generation of bispecifics and nanobodies/multispecifics, respectively.
Not Just Squeaking By
Animal model providers are part of a robust market, one that is projected to grow at a compound annual growth rate of 8.6% and reach a value of $24 billion by 2025. These figures come from Global Market Insights, which cites a range of market drivers including a proliferation of research applications, an upsurge in the development of new medicines, and rising demand for precision medicine.
Continued growth, then, depends on the market’s response to increasingly sophisticated demands. The main demand is for animal models that can represent the disease pathways seen in humans. However, there are other demands that merit attention. These emerged from a brief Q&A that GEN had with a couple of animal model experts—Sheryl Wildt, PhD, global manager of genetic quality and breeding at Envigo and Alexandre Fraichard, PhD, CEO of GenOway.
GEN: Which animal model factors deserve more attention?
Wildt: There are many factors to consider, including stock or strain, gender, age, and gut microflora. Use of historical data for model selection is an often-overlooked area and can make translation of the experimental results more evidence based, helping to refine the design of the experiment, and resulting in an adherence to the principles of the 3Rs—the replace, reduce, and refine approach to minimizing the use of animals and maximizing animal welfare.

Sheryl Wildt, PhD, Envigo
The genetic background of a model can be another unplanned variable. In addition to influencing research results, genetic background may dictate how well the animals breed.
Fraichard: The main challenge of preclinical studies is to understand the etiology of human diseases and design translatable models. For example, immunological disorder animal models must mimic human immune response.
It is important to clearly determine a development project’s aim and design the model accordingly: a model for target validation is different from one conceived to assess drug efficacy and safety. Preclinical models are often inbred lines and do not reflect human genetic variability: this must be considered when interpreting experimental data.

Alexandre Fraichard, PhD, GenOway
GEN: Which research areas are underserved by animal models?
Wildt: Over the past several decades, Alzheimer’s research has yielded a prodigious amount of promising results, but effective treatments in humans has remained elusive. Current mouse models of Alzheimer’s can recapitulate both the plaques and tangles seen in the disease pathology, but they fall short of demonstrating the full physiologic changes seen in humans as the disease advances. Advances in targeted gene editing techniques, such as CRISPR/Cas9, may allow for improved models that can more fully replicate the human disease pathology of Alzheimer’s disease.
Fraichard: The increasing prevalence of metabolic disorders poses an urgent need to develop models to study their etiology and develop new therapeutics. For example, the lack of reliable models for nonalcoholic fatty liver disease hinders the development of preventive and therapeutic strategies for this disease.
Recently, combination therapy and chimeric antigen receptor T-cell therapy have gained a momentum in immuno-oncology, showing promising results in a broad range of malignancies. GenOway has developed preclinical models to assess the efficacy and mechanism of actions of these new therapies.


