May 1, 2010 (Vol. 30, No. 9)
Next-Generation Products for Use with Neural, Hepatocyte, Chondrocyte, and Stem Cell Lines
The study of mammalian cells cultured in the lab has become an increasingly powerful research tool. As techniques and technology have improved, so too has the ability to obtain meaningful results—allowing researchers to make inroads into understanding the fundamental mechanisms of cell behavior.
Today, most types of mammalian cells, including stem cells, can be grown. There are limitations, however. Successful laboratory culture does not necessarily mean that cells grown in this way will behave in a natural in vivo manner. Even growing cells on a 3-D scaffold, instead of a 2-D surface made of the same material, can make a significant difference to their metabolic characteristics. For example, using hepatocytes, this is demonstrated by differential levels in key measures of hepatocyte function such as albumin secretion (Figure 1) and cytochrome P450 activity (Figure 2).
To initiate more characteristic behavior it is often necessary to provide cells with specific signals from the culture environment. The nature of these signals can be vitally important—even subtle changes in the growth media can have dramatic effects on cell behavior. This is one of the reasons there is widespread interest in moving away from growing cells in nondefined animal-based liquid media and toward the use of chemically defined synthetic alternatives.
In many cases, removing the animal-based components has reduced batch variability, allowing more consistent and meaningful experimental results.
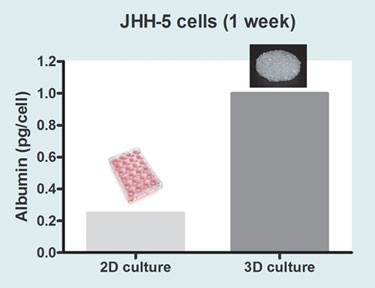
Figure 1. Comparison of albumin production levels in JHH-5 cells grown on OrlaClear, a high optical-grade 2-D borosilicate glass, and Orla CellCarrier, a 3-D porous scaffold, made from the same borosilicate glass material. The production of serum albumin is one of a number of functions that can be used to estimate how successful in vitro culture has been in inducing in vivo hepatocyte-like behavior. When a range of hepatocyte cell lines were tested, there was a clear increase in albumin production in 3-D-grown cells compared to 2-D.
Changing the media in which cells grow, however, is only part of the answer to providing a controlled and reproducible environment. The nature of the surface to which cells attach can also greatly affect their behavior. For example, most neural cells can not differentiate unless they are grown on a surface coated with an appropriate extracellular matrix material such as laminin. And different cells have different surface requirements. For some, simply coating the surface with positively charged material such as polylysine provides the necessary stimulation for cells to attach and prosper.
Other cell types, however, are more demanding and require complex biological coatings for successful culture. In the past, human embryonic stem cells have routinely been grown on feeder cells or surfaces coated with animal-derived extracellular matrix components. These biological surfaces have been effective in providing cells with a culture environment that mimics in vivo conditions, though their variability and propensity to contain unwanted contaminants such as growth factors has been a problem. Providing alternative growth environments for demanding cells such as these has not been easy—nevertheless, real progress is being made.
The first generation of animal-free, bio-active growth surfaces ranged from products containing relatively simple, small synthetic peptide domains to those featuring whole multi-unit proteins produced and subsequently purified from mammalian cell culture. These proteins were designed to mimic extracellular matrix components and could be coated on a variety of surfaces.
The random nature of protein attachment and presentation with first-generation products meant that only a small percentage of the active domains of these proteins were accessible and correctly displayed for interaction with cells cultured on the surface. Aside from hindering experimental efficiency, this was financially wasteful, as only a limited number of these expensive proteins was available to deliver the desired biological stimulus.
Next-generation products such as those available from Orla Protein Technologies help overcome these deficiencies. For the Orla system, a specific cell wall protein was selected and carefully engineered to auto-adhere to surfaces such as glass. It also adheres to gold, enabling parallel protein-binding studies using techniques such as SPR and QCM.
The protein has enhanced beta-barrel rigidity to aid membrane formation and upper-end peptide sequences into which peptides or protein motifs can be inserted for presentation to cells within a natural loop feature.
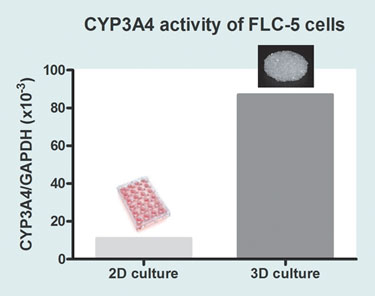
Figure 2. Comparison of CYP-3A4 mRNA levels in FLC-5 cells grown on 2-D borosilicate glass and Orla CellCarrier, a 3-D porous scaffold made from the same glass. Because cytochrome P450-3A4 (CYP-3A4) in the liver is heavily involved in the processing and removal of many pharmaceutical agents, it is essential that any hepatocyte model system used in pharmaceutical research or toxicological testing express appropriate levels of CYP-3A4. Cells grown on 3-D CellCarrier show a large increase in CYP-3A4 mRNA compared with cells grown on an equivalent 2-D surface.
The protein system auto-assembles from aqueous solution into “membranes” that are both stable and sterilizable, with a dense protein monolayer where every protein molecule is in the correct orientation. Chosen concentrations of peptide motifs are achieved by using “filler” molecules for dilution and to fill any remaining membrane gaps. The filler molecules can be hydrophobic, hydrophilic, or functionalized to enable the patterning or printing of surfaces. For example, the use of a PEG filler will inhibit cell adhesion, enabling scientists to examine cell adhesion and mobility characteristics.
As a result, scientists can present cells with peptide motifs in any concentration, mixture, or ratio—on surfaces and media that are animal-free and fully characterized and with proteins presented to cells in the correct orientation as well as in a naturalistic protein loop manner.
These next-generation products overcome variability issues. Their innate reproducibility enables scientists to investigate the ideal growth conditions for a chosen cell line—and then to apply meaningful assay parameters to understand cell behavior.
To date, these systems have been used with a wide range of mammalian cells (including neural, hepatocyte, chondrocyte, and other cell lines) and also human embryonic stem cells. The applications range from studies of cell adhesion, mobility, differentiation, and fate to the development of assay conditions allowing metabolic investigation and screening. A good example of this kind of behavior has been shown in recent adhesion studies done on neural cells (Figure 3).
It is already becoming clear that extracellular matrix proteins contain numerous domains and motifs, each of which can be a target for receptors expressed on the surface of cells. Even within a single protein, these motifs can be synergistic or antagonistic in effect. Sometimes the effect of using multiple motifs is predictable; in other cases a combination of motifs results in a totally different cell effect to that which might have been predicted from the study of individual motifs.
Nature is complex, and there is still a lot to be learned about cells. Nevertheless, the availability of well-characterized and affordable products facilitates the study of mammalian cell characteristics and helps to assemble the knowledge required to move toward research, development, and clinical solutions.
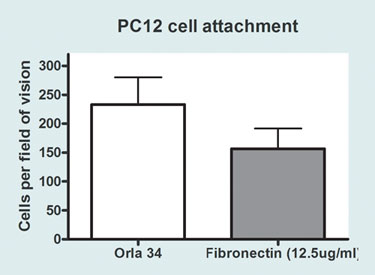
Figure 3. Comparing the attachment of PC12 cells grown on a fibronectin surface to an Orla 34 (fibronectin PHSRN motif) surface. PC12 cells (a neuron-like cell line) were grown on OrlaGold 2-D surfaces coated with ECM protein motifs, good cell adhesion was achieved compared with surfaces coated with the corresponding whole proteins. With differentiating PC12 cells: collagen I and fibronectin induced the formation of beta-III-tubulin positive cells, whereas collagen IV inhibited it. When multiple motifs were used, combinatorial effects could often be predicted. However, this was not always the case, demonstrating the importance of being able to compare different motifs individually and in different combinations.
Sion Phillips, Ph.D. ([email protected]), is a senior researcher at Orla Protein Technologies.



