June 15, 2013 (Vol. 33, No. 12)
Improving Adherent Cell Culture Performance on Microcarriers in Bioreactors
A common solution for producing higher quantities of anchorage-dependent cells has been to use large numbers of roller bottles and multi-trays to simply multiply the number of static surface areas.
The main disadvantage of this approach is that the process requires large operational space, cost, and time. With growing demand for large-scale production of vaccines, stem cells, and personalized medicines, more efficient and scalable production methods for anchorage-dependent cells has become necessary.
The suspension-culture method using micro-carriers in large vessels has been used as a more efficient and cost-effective way to scale-up this process. However, this approach has limitations.
Anchorage-dependent cells on microcarriers in large volume in bioreactors are more sensitive to hydrodynamic shear stress than are suspension cells. To maintain constant mass transfer efficiency in traditionally designed stirred bioreactors, shear stress increases as the size of the vessel increases. This makes the scaleup of the shear-sensitive process challenging.
An ideal bioreactor for microcarrier-based cell-culture processes would provide high mass transfer and good mixing but without the attendant high hydrodynamic forces—regardless of the size of the reactor.
The initial seeding phase of anchorage-dependent cells onto microcarriers is particularly sensitive to hydrodynamic forces. Until the cells are securely anchored to the surface of micro-carriers with cell-attachment proteins, the cell attachments are relatively weak and susceptible to hydrodynamic shear damages.
In many cases, this attachment step is carried out in static conditions coupled with brief, intermittent agitations, because the shear stress caused by impeller agitation inhibits cell attachment.
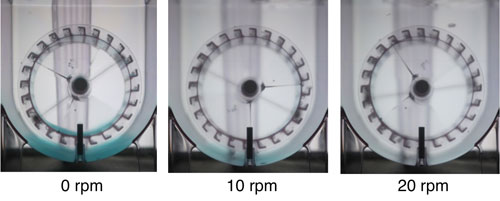
Figure 1. Microcarrier suspension in 3 L Air-Wheel bioreactor: Cytodex-1 microcarriers were stained with blue ink and mixed in the bioreactor at different agitation rates to determine the minimum agitation rate required for microcarrier suspension. The left image shows microcarrier sedimentation with no agitation. While 10 rpm agitation rate resulted in good suspension, there was still some intermittent settling of microcarrier beads. Full microcarrier suspension with no settling was achieved at 20 rpm.
Improved Mixing Mechanism for Scalable Bioreactor Functions
The Air-Wheel® single-use bioreactor from PBS Biotech utilizes a large, vertically oriented impeller to promote efficient particle suspension at relatively slow agitation speeds. The buoyancy of sparged gas is converted into rotational energy by the Air-Wheel impeller, providing short mixing times and good mass transfer rates at very low levels of hydrodynamic shear stress.
Mixing is achieved by broad, opposing vanes on the impeller that create a cut-and-fold flow pattern and dissipate mechanical energy homogeneously throughout the liquid culture medium. In addition, a strong, tangential flow, along with the “U”-shaped bioreactor vessel, provides uniform suspension of microcarriers inside the vessel.
Computational fluid dynamics modeling shows that not only are the average wall shear rates in Air-Wheel bioreactors much lower than those found in traditional stirred bioreactors at each scale, but they are at a constant low rate across all scales.
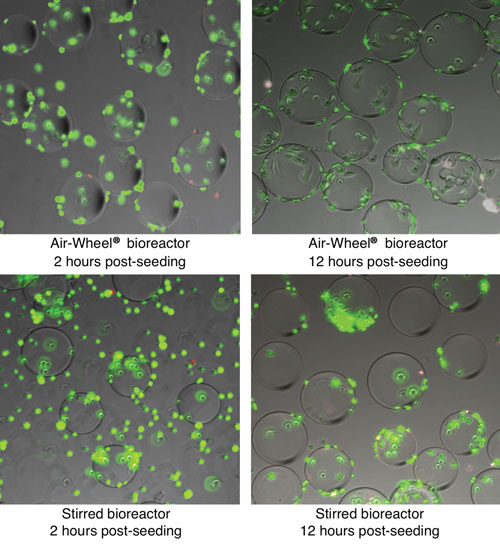
Figure 2. Kinetics of cell attachment: The images in the top row represent samples taken from the Air-Wheel bioreactor at two hours and twelve hours post-inoculation time points. The images in the bottom row represent samples taken from the traditional stirred bioreactor at identical time points. Fast, even attachment of cells on microcarriers is evident in the Air-Wheel system.
To test the kinetics of cell attachment and cell growth performance on microcarriers in the Air-Wheel bioreactor, human alveolar adenocarcinoma (A549) cells were cultured on 3 g/L Cytodex-1 micro-carriers from GE Healthcare Life Sciences at Instituto de Biologica Experimental e Tecnologica (IBET) in Portugal. The cells were cultured for up to 150 hours using FK12 medium (Invitrogen) supplemented with 10% fetal bovine serum.
As a control, a 200 mL stirred bioreactor with a four blade marine impeller was run in parallel under the same conditions but at 90 rpm, a typical impeller agitation rate for this system. A preliminary mixing experiment determined that the minimum agitation rate to achieve homogenous microcarrier suspension in the Air-Wheel bioreactor was 20 rpm (Figure 1).
To compare the effects of shear stress on cell attachment kinetics, cells were added to traditional and Air-Wheel bioreactors containing Cytodex-1 microcarriers and continuously stirred during seeding. Samples were taken and examined microscopically and analyzed for cell growth measurement.
Cell Attachment and Growth on Microcarriers
As shown in Figure 2, cell attachment occurred much more quickly and evenly in the Air-Wheel bioreactor than in the traditional stirred bioreactor. In the Air-Wheel system, most of the cells attached evenly on micro-carriers just two hours after seeding and showed the spread morphology indicative of healthy cell attachment after only 12 hours.
In the traditional stirred bioreactor, good cell attachment was not observed until after 12 hours, and even then was found to be nonuniform.
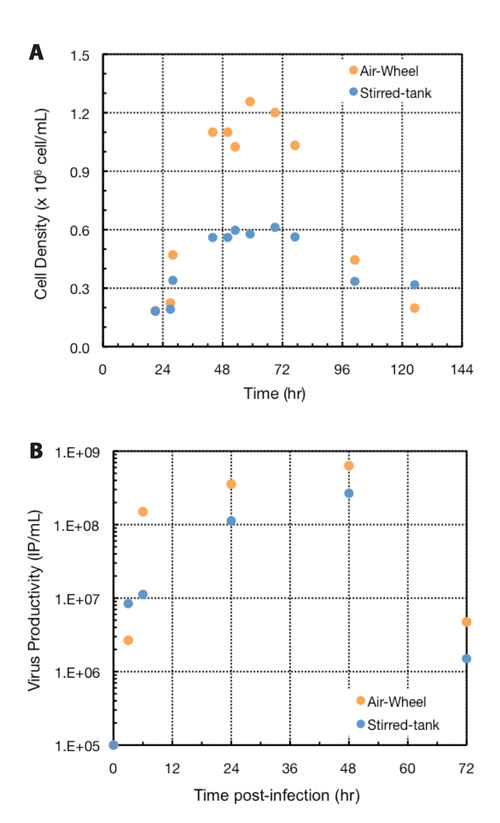
Figure 3. Cell density and virus productivity: Viable cell density (A) and virus productivity (B) data was measured on samples taken from both the Air-Wheel and traditional stirred bioreactors. After detachment of cells from the microcarriers, viable cell density was counted on a hemacytometer after staining with Trypan blue. Virus productivity was quantified using the 50% Tissue Culture Infectious Dose (TCID50) method. Both cell density and productivity were found to be twice as high in the Air-Wheel bioreactor compared to traditional stirred bioreactor.
Samples taken aseptically from the bioreactors at intermittent time points also revealed faster cell growth rates and twice the peak cell density in the Air-Wheel bioreactor at the same seeding density (Figure 3A). This data confirms the results from visual observation under the microscope that cells reached confluence on microcarriers and attained the desired spread morphology faster in the Air-Wheel system, a clear indication that cells were healthier in this bioreactor.
Virus Productivity
The A549 cells were infected with oncolytic adenovirus at 50 hours post-seeding once confluence was reached on microcarriers, and virus particle titer was quantified. Both intracellular and extracellular volumetric viral productivity were found to be higher in the Air-Wheel system than in the stirred bioreactor (Figure 3B).
Superior cell growth and viral productivity achieved in the Air-Wheel bioreactor system was credited to its low-shear mixing and homogenous particle suspension environment that allowed cells to attach to micro- carriers faster and more evenly.
In summary, the single-use bioreactor system from PBS Biotech with patented Air-Wheel vertical mixing technology offers efficient, homogenous, and scalable liquid mixing in a low-shear environment. These characteristics enable improved performance of anchorage-dependent cell cultivation in suspension, and allow process to scale up to large production scales in a faster, more productive, reliable, and cost-effective way.
Yas Hashimura, is director, applications engineering, Daniel Giroux is vp, R&D, and Brian Lee, Ph.D. ([email protected]) is president at PBS Biotech. The authors would like to thank Marcos Sousa, Joao Clemente, Dr. Paula Alves, and Dr. Manuel Carrondo at the Instituto de Biologia Experimental e Tecnologica (IBET), Lisbon, Portugal, for their experimental work.