July 1, 2011 (Vol. 31, No. 13)
Firm Increasing Offerings and Developing New Ways to Conjugate and Label Peptides
For nearly 25 years, American Peptide has manufactured high-quality peptides. The company started in 1987 as a contract manufacturer of custom and catalog peptides in Sunnyvale, CA, and in 2002, opened a 34,000 sq. ft. facility in Vista, CA, that specializes in manufacturing cGMP and pharmaceutical-grade peptides. The company was acquired by Japan’s Otsuka Chemical in 2008, but operates under its original name.
In the last three years alone, American Peptide has produced 20,000 peptides, and 200 of them have progressed to be active pharmaceutical ingredients (API). “We have extensive experience in manufacturing milligram to multikilogram quantities and in conjugation and PEGylation technologies,” says organic chemist Firuz Shakoori, director of sales.

Phalloidin is a bicyclic 7 amino acid peptide toxin originally isolated from the poisonous mushroom Amanita phalloides. Phalloidin binds specifically at the interface between F-actin subunits.
Synthetic Phalloidin
The company continues to develop new peptides, as well as new ways to conjugate and label peptides for research and clinical use. Among the new products in the upcoming 2012–2013 catalog will be a synthetic phalloidin. This bicyclic, 7-amino acid peptide toxin binds specifically at the interface between F-actin subunits, where it stabilizes actin filaments, promotes actin polymerization, and blocks the conversion of F-actin polymers to G-actin monomers.
Phalloidin originally was isolated from the poisonous mushroom Amanita phalloides. The synthetic phalloidin is conjugated to biotin or fluorescent dyes to precisely label actin filaments. Researchers use phalloidin to study cellular processes involving actin, such as tumor formation, neurodevelopment, birth defects, and tissue regeneration. “The synthetic form is easier to use and cheaper to work with than natural phalloidin,” says Shakoori.
Heavy Isotopes
Custom peptides labeled with heavy isotopes are another expanding area. Researchers use heavy isotopes to identify and develop novel biomarkers for diagnostic and therapeutic applications. Carbon, hydrogen, and nitrogen, three elements naturally abundant in proteins, exist in light and heavy forms. For instance, 13C has the same number of protons and electrons as 12C and behaves the same chemically, yet it has one extra neutron that makes it heavier. Similarly, 2H and 15N are heavy isotopes of hydrogen and nitrogen.
About 1% of natural proteins consist of these heavy isotopes. However, heavy isotopes can be artificially incorporated into synthesized peptides and exploited in various ways, most notably as internal standards for protein quantification by mass spectrometry.
Biomarkers, allergens, and other clinically important proteins are present in minute quantities in complex biological mixtures like cell lysates, making them difficult to detect and quantify. But once a peptide labeled with stable isotopes is generated, the ratio of light native peptide to heavy isotopic peak intensities provides a relative measure of protein abundance. This permits numerous proteins to be evaluated simultaneously in biological samples.
“We make custom heavy isotope peptides via customer request by cold labeling amino acid derivatives and inserting them into sequences,” says Shakoori.
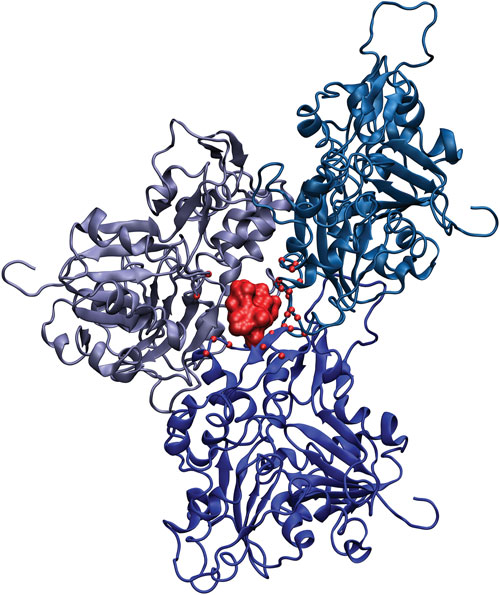
An actin trimer with bound phalloidin (colored red): Amino acid residues in contact with phalloidin are shown with red spheres.
PEGylation Expertise
Peptide drugs are increasingly complex and require challenging modifications, such as incorporating unnatural amino acids or being linked to carrier molecules to enhance drug delivery and efficacy. Although peptides have tremendous therapeutic potential, they often are difficult to synthesize and retain activity.
The scientists at American Peptide reportedly excel at PEGylation technology, which attaches chains of polyethylene glycol (PEG) to improve drug performance. The benefits of PEGylation include decreased immunogenicity, increased half-life in vivo, and reduced renal clearance.
PEG can also act as a repellent to prevent bacteria from contaminating medical implants and catheters. Grafting PEG to plastics and metals is challenging and requires linking it to hydrophobic molecules. “We have good expertise at making hydrophobic peptides,” says Shakoori. These peptides contain high concentrations of hydrophobic amino acids, such as leucine, isoleucine, and alanine, which are insoluble and hard to purify.
Clients come to American Peptide in various stages of the drug development process, but most are in Phase I and II. They receive help with stability, solubility, degradation, and impurity issues. Other services include process development, scale-up production, analytical and process validation, stability studies, and regulatory support. As genome mapping information increases, clients are able to find and develop specific peptides that target a variety of diseases.
Custom and catalog peptides make up about 40% of the company’s business, and cGMP and API manufacturing form the other 60%. The cGMP business is thriving and expected to grow even more as more peptide therapeutics advance through Phase II and III trials. American Peptide works with customers to validate lots, submit a drug master file, and eventually make the drug after FDA approval.
American Peptide takes pride in making all components of its cGMP peptides in-house. “All of the small-to-large scale and solid-phase to solution-phase processes are done under the same roof at Vista,” says Shakoori.
Although, some peptide manufacturers contract out stages of production to Chinese or European facilities, keeping all activities in-house provides better quality control because the same rules apply to all steps of production, according to American Peptide.



