May 15, 2013 (Vol. 33, No. 10)
Reactive oxygen species (ROS) are generated as natural byproducts of the cellular metabolism. They are involved in various biological processes and act as important signal mediators. However, excess intracellular levels of ROS may result in cell and tissue damage and are therefore associated with degenerative diseases, most notably cancer. In healthy individuals, intracellular antioxidant systems serve to maintain ROS levels below a critical threshold, permitting essential ROS-mediated signalling processes to function, but preventing ROS overproduction and potential tissue damage. Cells that fail to compensate and neutralize heightened ROS levels die by apoptosis in order to avoid passing on ROS-caused DNA damage to daughter cells. Any dysfunction in the cellular antioxidant systems can have serious consequences1.
In addition to the cells’ own antioxidant systems, various studies have suggested a relationship between an antioxidant-rich diet and a good health status, implicating that the consumption of antioxidant-containing foods can help to maintain health and even prevent certain diseases2.
A well-established and reliable method to determine the antioxidant capacity of a substance is the oxygen radical absorbance capacity (ORAC) assay3. It is based on the inhibition of oxyradical-induced oxidation of 2,2´-azobis-(2-methylpropionamidine) dihydrochloride (AAPH) by substances with antioxidant properties. Peroxyl radicals produced in a time-dependent manner during the thermal decomposition of AAPH will quench the fluorescence signal. In the presence of a substance with antioxidant properties the fluorescence reduction is inhibited, depending on the substance’s ORAC capacity. The dynamics of the signal inhibition, expressed as the area under the curve (AUC), is used to quantitate the antioxidant capacity, expressed as the ORAC value, by comparing the sample AUC to an antioxidant standard curve generated with Trolox, a water-soluble vitamin E-analogue.
This note describes the performance of the ORAC assay on the Infinite F200 PRO, using different beverages as antioxidant samples.
Material and Methods
The ORAC values of different beverages were determined in the Infinite F200 PRO, using the OxiSelect™ ORAC Activity Assay4. The following beverages were tested: apple juice, blackcurrant juice, grapefruit juice, orange juice, pineapple juice, red grape juice and red wine. The fruit juices and red wine were obtained from a local supermarket. Care was taken to purchase only 100% juices.
The OxiSelect ORAC Activity Assay was performed according to the manufacturer’s instructions4 in black 96- and 384-well plates (using adapted volumes). Since the results were similar in both plate formats, only data measured in 96-well plates is shown in this note. The standard curve was generated by diluting Trolox from 50–3.1 µM in PBS. The assay plates were covered with sealing film to minimize evaporation and incubated for 30 min at 37°C using the instrument’s “Incubation” function. Fluorescence measurements at 485(20) nm excitation and 535(25) nm emission were taken every 60 seconds over a total measurement period of 60–100 minutes. After the first three cycles (representing the baseline signal), AAPH was injected into each well to initiate the ROS generation. Depending on their individual antioxidant capacity, the tested samples were able to maintain the fluorescence signal on a high level for a certain period of time.

Results and Discussion
An antioxidant standard curve was generated using different concentrations of Trolox. Figure 1 shows the Trolox dose-dependent loss of fluorescence induced by the addition of AAPH. The measured fluorescence values were normalized to 100%, reflecting the initial fluorescence signals after the AAPH addition. The antioxidant capacity, expressed as the area under curve (AUC), was calculated for each Trolox concentration. The Net AUC values, reflecting the blank-corrected AUC values of the used standards, were plotted against the Trolox concentrations, and the linearity of the standard curve was calculated (Figure 1 inset).
During the analysis of the beverage samples, it became evident that some samples could not be analyzed in an undiluted state because they reduced the fluorescence signal even in the absence of the ROS-generating agent (AAPH). The individual fluorescence quenching potential was assessed for all samples using dilutions from 1:1 to 1:1000. Significant fluorescence quenching was observed in the 1:1 and 1:10 dilutions. Therefore, only 1:100 dilutions of the beverages were used in all further analyses.

Figure 1. (A) Signal curves measured with different Trolox concentrations (results normalized to initial fluorescence signals after AAPH addition). (B) Linearity of Trolox standard curve.
The ORAC values of the beverage samples were extrapolated from the Trolox standard curve. The analyzed beverages exhibited different signal kinetics and, thus, a different individual antioxidant capacity. The highest antioxidant potential was seen with red wine. Among the nonalcoholic beverages, blackcurrant and grapefruit juice were the most potent antioxidants, while orange and pineapple juice, respectively, showed the least antioxidant potential (Figure 2). Importantly, the ORAC values determined for the beverages are only valid for the tested products. Depending on the fruit content and composition, the ORAC values for beverages from other manufacturers may differ.
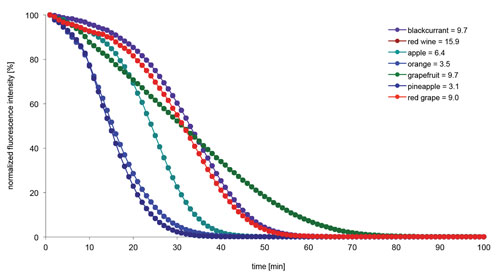
Figure 2. Signal curves of different beverage samples (all used in 1:100 dilution) and corresponding ORAC values expressed in µmol TE/mL.
Conclusion
The results summarized in this note demonstrate the excellent applicability of the Infinite F200 PRO for the ORAC assay. The instrument’s on-board injector system and the incubation function greatly facilitate the assay handling, making the Infinite F200 PRO ideally equipped for reliable and reproducible ORAC assays.
Tecan Austria
Untersbergstr.1a
5082 Groedig, Austria
Katrin Flatscher, Ph.D.
Application Scientist
Research and Development
[email protected]
www.tecan.com/detection
References:
1. Oberdanner C. ROS and Antioxidant Systems in Apoptosis. 2008
2. Slavin JL et al. Health benefits of fruits and vegetables. Adv Nutr. 2012. 1;3(4):506-16
3. Cao G et al. Oxygen-radical absorbance capacity assay for antioxidants. Free Radical Biol. Med. 1993.14: 303-311.
4. OxiSelect Oxygen Radical Antioxidant Capacity (ORAC) Activity Assay. Kit instructions. STA-345. Cell Biolabs Inc.



