September 15, 2016 (Vol. 36, No. 16)
Drug Developers Deplore the Gap between Conventional 2D Cell Cultures and In Vivo Animal Models
The costs associated with bringing a novel drug to market are exorbitant. According to a report issued by the Tufts Center for the Study of Drug Development, these costs exceed $2.5 billion per successful compound. The Tufts report also noted that drug development is lengthy, often taking longer than a decade. Consequently, researchers and pharmaceutical executives alike are anxious to increase the efficiency of drug development.
According to Tufts, drug developers are increasing efforts to discover, validate, and use biomarkers; adopting new approaches to patient recruitment and retention; and implementing leading-edge project management practices. Drug developers are also looking for better preclinical drug evaluation systems.
Historically, drug discovery research has relied on two-dimensional in vitro assays (that is, cell monolayers cultured on plastic substrata) and in vivo animal models. Although these systems still predominate, drug developers are beginning to doubt whether the familiar assays and animal models are adequate with respect to physiological relevance and clinical predictability. And drug developers are losing patience with persistently dismal rates of translation.
“There are some valid concerns that the in vivo models we rely on don’t really replicate the human response,” states Jason Ekert, Ph.D., senior research scientist, Janssen BioTherapeutics. “We need better in vitro cellular models to replicate what is happening in diseased human tissue.” Three-dimensional (3D) human cellular models of increasing complexity have emerged as a potential remedy to this deficit, and they could serve as a “bridge between animal testing and human clinical trials,” suggests Jeffery X. Cui, Ph.D., vice president of research and development, Stemorgan Therapeutics.
Although 3D cell culture promises to provide a more relevant in vitro model for drug screening, there is still work to be done to see how these results actually translate to the clinic. “We need to increase biological relevance but also predictability,” states Dr. Ekert. “This is an area where the data still needs to be collected and verified.”
That data is coming, asserts Paul Vulto, Ph.D., founder of Mimetas, an “organ on a chip” company. He points to a “revolutionary era” for predictive models in drug discovery. According to Dr. Vulto, a trend toward more complex, 3D culture models is sweeping the pharmaceutical industry. With ongoing advancements in 3D cell technology, the potential for noninvasive, physiologically relevant (human), high-throughput drug screening and biomarker profiling is being realized.

An inkjet bioprinting approach to creating bone and cartilage tissue has been reported by scientists based at Stemorgan Therapeutics. Tissue constructs demonstrating an improvement of mechanical properties and osteogenic and chondrogenic differentiation were created that incorporated poly(ethylene glycol) dimethacrylate, gelatin methacrylate, and human mesenchymal stem cells.
Accessible Kidney-on-a-Chip
Drug-induced nephrotoxicity is an abiding concern, but it still lacks reliable models. Better models, Mimetas suggests, may come from applications of 3D cell culture technology. One such application is Mimetas’ own kidney-on-a-chip system. Using advanced microfluidics technology, the Mimetas team is able to recreate structural and physiological properties of the human kidney. For example, in the Mimetas system, perfused proximal kidney tubules are grown in microfluidic channels in standard microplate format.
“The format is important,” explains Dr. Vulto. “The system needs to be as simple as possible. You shouldn’t need a Ph.D. in microfluidics to use our chips.” Moreover, the standardized plates are compatible with all microscopes, luminescent readers, and automation systems, which make it accessible in standard biological lab facilities as well as full-fledged high-throughput screening facilities.
In an effort to better recapitulate the in vivo situation, Mimetas researchers place their complex human kidney models into relevant microenvironments consisting of stromal cells, vasculature, and extracellular matrix molecules. Dr. Vulto acknowledges that “reproducing the 3D microenvironment is essential for developing physiologically relevant in vitro model systems in drug discovery.”
Dr. Vulto shares his vision for the future of these improved predictive models: “The next step is to generate the tissue models with an individual person’s own cells so that we can identify the negative side-effects and efficacy of a compound specifically for that individual prior to treatment.” The promise of personalized, predictive medicine in the organ-on-a-chip field is incentivizing researchers to close the technology acceptance gap, and driving pharmaceutical companies to fund the researchers’ studies.
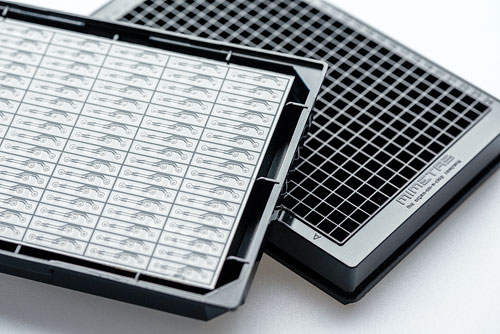
OrganoPlates, culture plates developed by Mimetas, incorporate passive liquid-handling technology for membrane-free definition of tissues in microfluidic chambers. Up to 96 tissue models can be supported on a single 284-well plate. The plate’s cell-encompassing gel supports 3D tissue configurations and cell-cell interactions. Continuous perfusion of media through the plate mimics blood flux and exchange of nutrients, oxygen, and metabolites.
Co-Cultured 3D Cardiomyocytes
Greg Luerman, Ph.D., head of application development at Axiogenesis, describes the development of what he calls a 2.5D platform to improve the physiological relevance of his firm’s cardiac cell culture model. Axiogenesis researchers create a co-culture by layering cardiac fibroblasts and myocytes (derived from induced pluripotent stem cells) in plates with a 2 µm-thin silicon membrane instead of a rigid bottom.
“By taking a multilayered cell system cultured in two dimensions and adding pressure and weight,” explains Dr. Luerman, “we get cardiac cells that beat more like a tissue, not only in the XY direction, but also the Z direction.” The force measurement that researchers are therefore able to record is a direct output of cardiac force, rather than a simple change in impedance.
“Interestingly, we have seen that adding in isogenic cardiac fibroblasts makes a huge difference in terms of force generation and inotropic response, especially when used in combination with ventricular-enriched cells,” Dr. Luerman continues. “These findings suggest that we are getting a much truer human cardiac phenotype than would be obtainable in a typical 2D system.”
The goal of this 3D model system is of course to lead us to predictive pharmacology, but Dr. Luerman is careful to point out that it isn’t just the added dimension that will get us there: “I don’t necessarily think we will see a huge jump from 2D to 3D models for a single cell type, but when you factor in the multi-cell-type systems, I think that is where we will begin to see a real difference.”
A Balancing Act for Tumor Spheroids
In an effort to better mimic the physiologic solid tumor environment, researchers at Janssen BioTherapeutics are investigating the applicability of spheroids in oncology drug discovery. Dr. Ekert describes the 3D tumor-like structures as “clusters of cells that self-assemble and provide a more biologically relevant tumor model than traditional 2D monolayer cultures.”
In fact, Janssen researchers have directly compared lung tumor cells grown as a 3D spheroid with 2D cultures and found important disparities. “Differences are evident in receptor density, phosphorylation, and cell proliferation both at baseline and in response to ligand,” states Dr. Ekert. “These changes are significant because they directly affect the cellular response to drug and growth factor treatment.”
Another consideration when incorporating these 3D models is that there is a balance between complexity, scalability, and automation. “Reducing the complexity of the system is sometimes necessary,” Dr. Ekert explains. “Doing so can achieve increased reproducibility and high throughput when screening large numbers of therapeutic candidates.” However, once the list of candidates has been narrowed, the technology also lends itself to more complex, multicellular studies aimed at validation and determining mechanism of action.
Novel Endpoints in 3D Culture
3D cell culture models are also opening new avenues for assay development by making previously inaccessible processes, including lumen formation and cell differentiation, available. “We are able to utilize 3D models to identify new targets that modulate processes that are very difficult or impossible to model on plastic,” asserts Aron B. Jaffe, Ph.D., senior investigator at Novartis Institutes for BioMedical Research. “This creates a space to study processes that were just not accessible with traditional 2D cell cultures.”
Specifically, primary progenitor cells from human conducting airways form a lumen when grown in three dimensions and differentiate into secretory goblet and ciliated cells. “We can then screen for modulators of goblet cell production,” states Dr. Jaffe. His team can mimic “asthma” in the 3D cultures with IL13, a cytokine found in about half of asthmatics. Although IL13 treatment is not unique to this model, the 3D format is conducive to assessing goblet cell differentiation, providing another physiologic measure for studying asthma and chronic obstructive pulmonary disease.
Dr. Jaffe also describes how the Novartis team identifies modulators of epithelial barrier function by essentially inducing diarrhea in a 3D intestinal model: “Human intestinal epithelial cells are grown in a 3D matrix where they form a structure that has an architecture similar to one found in the human gut—a single layer of cells surrounding a fluid-filled lumen. When treated with cholera toxin, the cells swell like a balloon, recapitulating aspects of secretory diarrhea in vivo.” This provides a physiologically relevant model to study drugs that may combat diarrhea or modulate the epithelial barrier.
Also significant for the 3D platform is the ability to adapt to high-throughput screening. “Very quickly we scaled our 3D model systems to 384-well plates for testing large numbers of therapeutic targets,” reports Dr. Jaffe. And the system, he adds, is easily adaptable to both small molecules and biologic targets, as evidenced by the successful screening of the entire human secretome, which comprises nearly 4,000 proteins. “We are more limited on the number of cells we can obtain when using primary human cells, than the 3D cell system itself,” explains Dr. Jaffe.
3D cultures are therefore providing more options for researchers interested in high-throughput compound profiling of morphologic and other, previously elusive endpoints.
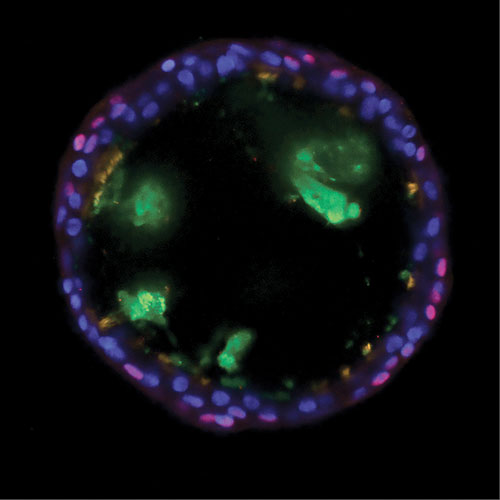
Scientists at the Novartis Institutes for BioMedical Research investigated basal cells and how they contribute to asthma by giving rise to unbalanced quantities of mucus-producing goblet cells and cilated cells. Using bronchosphere models, the scientists discovered that inhibiting a protein called Notch2 shifts the balance of cell production toward ciliated cells. The healthy bronchosphere in this image shows basal cells (red), mucus (green), ciliated cells (orange), and DNA (blue).
Applicability of 3D Bioprinting
Thermal, water-based inkjet bioprinting, which promises new capabilities for tissue engineering but also drug discovery, is probably the most highly touted technology for building tissues in three dimensions. With its potential to precisely recreate the native anatomic structure of human tissues, bioprinting has an almost science fiction-like allure for mainstream audiences. Limitations do exist, however, and thus far researchers have had difficulty printing asynchronous or thick tissues that require vascularization. “We need to understand the vasculature better to be able to recreate that network,” explains Dr. Cui. “This is a problem not just for bioprinting, but for 3D tissue engineering in general.”
Dr. Cui and colleagues have had success printing human tissues such as skin, cartilage, and bone—tissues that harbor therapeutic potential for burn repair and other injuries. As far as future indications for bioprinting are concerned, Dr. Cui envisions “a portable, handheld printer that can be utilized in the operating room to scan the surface of the joint, for example, and then print out a personalized orthopedic for the individual undergoing surgery.”
Generating personalized tissues is not the only potential for bioprinting. Producing automated, high-throughput, identical micro-organs could also have a big impact for drug discovery. “Building micro-organs and other 3D cellular models in multiple-well plates with a bioprinter is advantageous because we can guarantee the number of cells placed in each well,” notes Dr. Cui. “It’s more precise compared to the other methods available on the market. We can also achieve a level of organization and complexity that may be more difficult to obtain with traditional cell culture methods.”