Transposons threaten genetic chaos during sperm and egg production, when the epigenome effectively drops its guard. Ordinarily, the epigenome carries DNA methylations, chemical markers that inactivate pieces of DNA, whether they are genes or transposons. During sperm and egg production, however, DNA methylations are temporarily erased, leaving the genome vulnerable.
Fortunately, reproductive cells have another line of defense—the endosiRNA system. endosiRNA refers to endogenous small interfering (si) RNA molecules, which are created by the cells in response to the RNA “messages” released by transposons. The endosiRNAs then act like a trap, allowing a protein called Argonaute2 (Ago2) to seek and destroy transposon messages before they cause any harm.
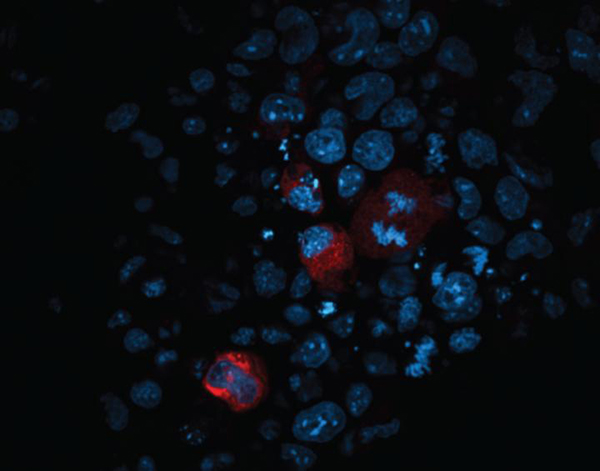
The connection between endosiRNAs and the suppression of transposon activity was recently discovered by scientists based at the Babraham institute. Because mammalian germline cells are hard to study, the scientists began their study by using mouse embryonic stem cells (ESCs) that had been genetically modified to lack DNA methylations. These cells mimicked the natural epigenetic reprogramming that occurs in primordial germ cells. Ultimately, the researchers used primordial germ cells to verify the key results from their study of stem cells.
Detailed results appeared November 2 in the journal Cell Stem Cell, in an article entitled “An endosiRNA-Based Repression Mechanism Counteracts Transposon Activation during Global DNA Demethylation in Embryonic Stem Cells.” The article described an “immediate” endosiRNA-dependent response, as well as a subsequent “chronic” response.
“In ESCs with DNA demethylation induced by acute deletion of Dnmt1, we saw an increase in sense transcription at TEs [transposable elements], resulting in an abundance of sense/antisense transcripts leading to high levels of ARGONAUTE2 (AGO2)-bound small RNAs,” wrote the article’s authors. “Inhibition of Dicer or Ago2 expression revealed that small RNAs are involved in an immediate response to demethylation-induced transposon activation, while the deposition of repressive histone marks follows as a chronic response.”
The Babraham team confirmed that TE-specific endosiRNAs are present during primordial germ cell development in vivo. Essentially, the Babraham team found that endosiRNAs do act like a trap, allowing a protein called Argonaute2 (Ago2) to seek and destroy transposon messages before they cause any harm.
“Epigenetic reprogramming plays a vital role in wiping the genome clean at the start of development, but it leaves our genes vulnerable,” said the paper’s lead author, Rebecca Berrens. “Understanding the arms race between our genes and transposon activity has been a long-running question in molecular biology. This is the first evidence that endosiRNAs moderate transposon activity during DNA demethylation. EndosiRNAs provide a first line of defense against transposons during epigenetic reprogramming.”
The effects of active transposons vary; often they have no effect and only occasionally will they alter an important gene. Yet, transposons can affect almost any gene, potentially leading to different kinds of genetic disease. Studying the control of transposons, the Babraham Institute noted, adds to our understanding of the many ways that they can impact on human health.
Transposons sit within genes and are read in the opposite direction to the surrounding gene. It is this arrangement that allows cells to identify RNA messages from transposons. RNA messages read from the same piece of DNA in opposite directions are complementary, meaning they can join to form a structure called double-stranded RNA (dsRNA), which initiates the creation of endosiRNAs.
“Transposons make up a large part of our genome, and keeping them under control is vital for survival,” explained the paper’s senior author, Wolf Reik, M.D., head of the Epigenetics Laboratory at the Babraham Institute. “If left unchecked their ability to move around the genome could cause extensive genetic damage. Understanding transposons helps us to make sense of what happens when they become active and whether there is anything we can do to prevent it.”