Scientists in Singapore have developed a bacterial protein nanoparticle that correctly folds recombinant proteins, increases functional expression yields up to 100-fold, and shields the internalized proteins from damage by heat, chemicals, and proteolysis. Scientists at the National University of Singapore (NUS), and the Nanyang Technological University, who developed the thermostable exoshell (tES), hope that the technology could help to address some of the problems with recombinant protein production, both for research and industrial applications.
“Our findings highlight the potential of using highly engineered nanometer-sized shells as a synthetic biology tool to dramatically affect the production and stability of recombinant proteins,” claims lead researcher Chester Drum, M.D., Ph.D., assistant professor at the departments of medicine and biochemistry, at NUS Medicine, who is also a consultant cardiologist at the National University Hospital and director of the Clinical Trial Innovation Lab at the Translational Laboratory in Genetic Medicine (TLGM), A*STAR.
The team reports on development of the tES in Nature Communications (“Thermostable Exoshells Fold and Stabilize Recombinant Proteins”).
Expressing and stabilizing functional recombinant proteins remains a key challenge for both basic and industrial biology, the authors note. Technologies for improving protein folding, which range from chaperone co-expression to chemically engineered hydrogels, have had “varied success.” Methods for stabilizing protein products, including chemical cross-linking, rational mutagenesis, and directed evolution approaches, have also been applied both for basic and industrial applications.
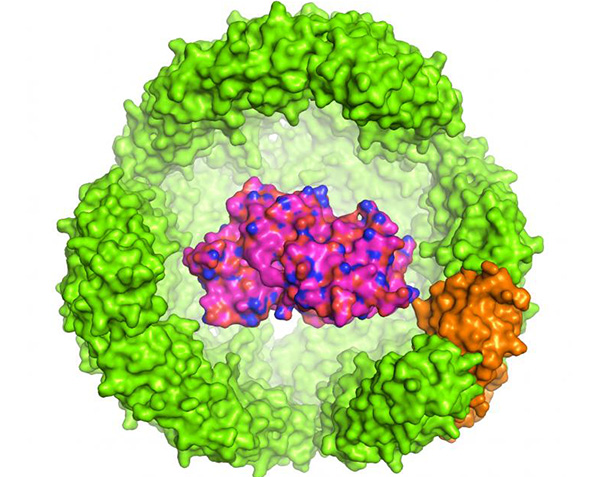
In contrast with existing approaches, Dr. Drum’s team aimed to develop a single technology that could both improve recombinant protein expression and product stabilization. To do this, they turned to Archaeoglobus fulgidus, a bacterium that lives in hydrothermal vents. Like eukaryotes, A. fulgidus produces an iron-carrying protein, ferritin (AfFtn), which is composed of 24 subunits. But unlike typical eukaryotic ferritin, the AfFtn shell contains four nanopores that give small molecules access to the interior of the shell. And while eurkaryotic ferritin is stable at low salt concentrations, AfFtn dissociates at low salt concentrations, so release of the tES contents can be triggered by changing the pH and applying proteolytic enzymes.
The researchers engineered the AfFtn into a tES that can hold foreign proteins inside an 8-nm aqueous cavity. The team aimed to test the effects of tES on the expression and folding of three recombinant proteins expressed in Escherichia coli. They prepared genetic fusions (tES-POI) between a tES monomer and one of three proteins of interest (POIs): green fluorescent protein (GFP), Renilla luciferase (rLuc), and a truncated version of horseradish peroxidase (HRP). All three POIs were chosen because they have traditionally proven tricky to express in E. coli or don’t fold properly. The researchers then co-expressed the tES-POI fusion and the tES shell together.
Initial analyses indicated that each tES-POI subunit was surrounded by a shell of additional tES subunits to form the complete encapsulating shell: “the ratios of tES-GFP, tES-HRP, and tES-rLuc to encapsulating tES subunits were in approximate agreement with the expected value of 1:23,” the team writes. The results also confirmed that the exoshell boosted functional yields of all three POIs, up to 100-fold in the case of GFP. “We hypothesize that the significant increase in functional protein yield may be due to the complementation between the negatively charged proteins and the positively charged exoshell internal surface,” Drum suggests.
And in all cases, expression of tES was needed for functional in vitro folding of the tES-POI fusions. In particular, the researchers were able to deliver the cofactors calcium and heme, and apply oxidizing conditions that ensured the correct folding and functioning of the complex horseradish peroxidase protein.The four pores in the shell gave internalized enzymes access to molecular substrates so that they can continue their catalytic functions.
The exoshells in addition protected the proteins against heat and chemical denaturing agents, including including high-concentration trypsin, organic solvents, and urea. “By using 23 copies of a single thermostable subunit to form a protective shell around internalized proteins, we report that tES can improve expression, in vitro folding, and product stabilization,” they conclude. “The ability to stabilize thermolabile substrates within tES may be helpful for a variety of applications in bionanotechnology and synthetic biology, including the production of difficult-to-fold proteins, using the shell as a mediator of cellular enzyme uptake, and exploiting the stabilized qualities of tES substrates in industrial settings.”
The researchers suggest that although about 80% of translated eukaryotic proteins are 80 kDa or smaller—the tES cavity is 8 nm—developing tES variants with larger internal volumes will expand use of the technology even further, particularly for proteins that have a multimeric functional structure.