![Mouse embryonic stem cells (ESCs) and extraembryonic trophoblast stem cells (TSCs) have been combined in a 3D scaffold to generate structures whose morphogenesis is remarkably like that of natural embryos. (Left) Stem cell-modeled mouse embryo at 96 hours; (right) mouse embryo cultured <i>in vitro</i> for 48 hours from the blastocyst stage. The red part is embryonic; the blue, extraembryonic. [Sarah Harrison and Gaelle Recher, Zernicka-Goetz Lab/University of Cambridge]” /><br />
<span class=](https://genengnews.com/wp-content/uploads/2018/08/Mar3_2017_UnivCambridge_StemCellModeledMouseEmbryo2491321313-1.jpg) Mouse embryonic stem cells (ESCs) and extraembryonic trophoblast stem cells (TSCs) have been combined in a 3D scaffold to generate structures whose morphogenesis is remarkably like that of natural embryos. (Left) Stem cell-modeled mouse embryo at 96 hours; (right) mouse embryo cultured in vitro for 48 hours from the blastocyst stage. The red part is embryonic; the blue, extraembryonic. [Sarah Harrison and Gaelle Recher, Zernicka-Goetz Lab/University of Cambridge]
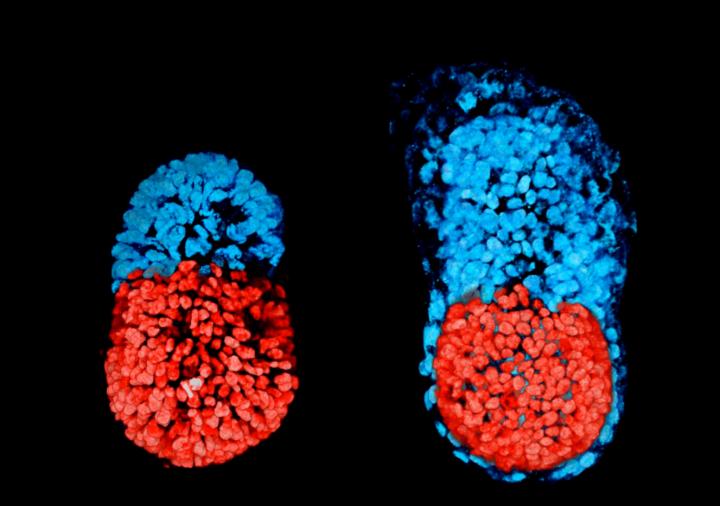
Mouse embryonic stem cells (ESCs) and extraembryonic trophoblast stem cells (TSCs) have been combined in a 3D scaffold to generate structures whose morphogenesis is remarkably like that of natural embryos. (Left) Stem cell-modeled mouse embryo at 96 hours; (right) mouse embryo cultured in vitro for 48 hours from the blastocyst stage. The red part is embryonic; the blue, extraembryonic. [Sarah Harrison and Gaelle Recher, Zernicka-Goetz Lab/University of Cambridge]
An artificial mouse embryo created by University of Cambridge scientists has all its parts in all the right places, except that it has a cavity where its yolk sac should be. And so it recapitulates many of the developmental milestones that are achieved by natural embryos, except that without a yolk sac, it can develop only so far. Regardless, the artificial embryo has embryonic stem cells (ESCs) at one end and extraembryonic trophoblast stem cells (TSCs) at the other.
In natural embryos, ESCs eventually give rise to the body, and TSCs eventually give rise to the placenta. Although the artificial embryo has properly positioned ESCs and TSCs, as well as the benefit of a 3D scaffold, or extracellular matrix, it is not likely to progress much beyond the post-implantation stage. Besides lacking the cells that would give rise to a yolk sac, the artificial embryo is not optimized for the correct development of the placenta.
Yet even young embryos, in convenient and ample supply, would be a boon to embryonic research. For example, they could help scientists understand why human development sometimes goes wrong or fails altogether. At present, more than two out of three human pregnancies fail at this early stage.
Details about the artificial embryo appeared March 2 in the journal Science, in an article entitled, “Assembly of Embryonic and Extra-Embryonic Stem Cells to Mimic Embryogenesis In Vitro.” The article describes how the University of Cambridge scientists succeeded where earlier embryo-construction efforts failed. Essentially, the scientists cultivated interactions between different types of stem cells.
“By using genetically-modified stem cells and specific inhibitors, we show embryogenesis of ESC- and TSC-derived embryos, ETS-embryos, depends on crosstalk involving Nodal signaling,” wrote the article’s authors. “When ETS-embryos develop, they spontaneously initiate expression of mesoderm and primordial germ cell markers asymmetrically on the embryonic and extra-embryonic border, in response to Wnt and BMP signaling.”
The University of Cambridge scientists, under the leadership of Magdalena Zernicka-Goetz, Ph.D., a professor of physiology, development and neuroscience, found a remarkable degree of communication between the ESCs and TSCs. In a sense, the cells tell each other where in the embryo to place themselves.
“Both the embryonic and extraembryonic cells start to talk to each other and become organized into a structure that looks like and behaves like an embryo,” explained Prof. Zernicka-Goetz. “It has anatomically correct regions that develop in the right place and at the right time.
“We knew that interactions between the different types of stem cell are important for development, but the striking thing that our new work illustrates is that this is a real partnership—these cells truly guide each other. Without this partnership, the correct development of shape and form and the timely activity of key biological mechanisms doesn't take place properly.”
Comparing their artificial embryo to a normally developing embryo, the team was able to show that its development followed the same pattern of development. The stem cells organize themselves, with ESCs at one end and TSCs at the other. A cavity opens then up within each cluster before joining together, eventually to become the large, so-called proamniotic cavity in which the embryo will develop.
While this artificial embryo closely resembles the real thing, it is unlikely that it would develop further into a healthy fetus, say the researchers. To do so, it would likely need the third form of stem cell, which would allow the development of the yolk sac, which provides nourishment for the embryo and within which a network of blood vessel develops.
Prof. Zernicka-Goetz recently developed a technique that allows blastocysts to develop in vitro beyond the implantation stage, enabling researchers to analyze for the first time key stages of human embryo development up to 13 days after fertilization. She believes that this latest development could help them overcome one of the main barriers to human embryo research—a shortage of embryos. Currently, embryos are developed from eggs donated through in vitro fertilization (IVF) clinics.
“We think that it will be possible to mimic a lot of the developmental events occurring before 14 days using human embryonic and extraembryonic stem cells using a similar approach to our technique using mouse stem cells,” she noted. “We are very optimistic that this will allow us to study key events of this critical stage of human development without actually having to work on embryos. Knowing how development normally occurs will allow us to understand why it so often goes wrong.”