![Overactive protein synthesis found in premature aging disease may also play role in normal aging. Nucleoli in the cell nucleus, stained bright magenta and cyan against the purple backdrop of the nucleus, are enlarged in the progeria cell (right) compared to the normal cell (left). [Salk Institute]](https://genengnews.com/wp-content/uploads/2018/08/149099_web2521183182-1.jpg)
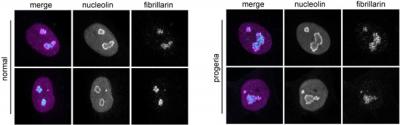
Overactive protein synthesis found in premature aging disease may also play role in normal aging. Nucleoli in the cell nucleus, stained bright magenta and cyan against the purple backdrop of the nucleus, are enlarged in the progeria cell (right) compared to the normal cell (left). [Salk Institute]
Premature aging is a condition that no one likes thinking about. So much so that the cosmetic industry has built a multi-billion-dollar empire of products that allegedly slow the aging process. Sadly, there are rare genetic conditions that cause rapid, premature aging, for which there are no creams, tinctures, or cures. However, these rare disorders may provide insight into the normal cellular aging process, leading to improved therapies for diseases such as Alzheimer’s, Parkinson’s, and the genetic aging disorder in Hutchinson-Gilford progeria syndrome (HGPS).
Now, investigators at the Salk Institute have uncovered an errant protein process in HGPS that could help healthy people, as well as progeria sufferers, live longer. Findings from the new study—published today in Nature Communications in an article entitled “Nucleolar Expansion and Elevated Protein Translation in Premature Aging”—showed that protein synthesis is overactive in people with progeria. These new findings add to a growing body of evidence that reducing protein synthesis can extend lifespan—and thus may offer a useful therapeutic target to counter both premature and normal aging.
“The production of proteins is an extremely energy-intensive process for cells,” explained senior study investigator Martin Hetzer, Ph.D., vice president and chief science officer at the Salk Institute. “When a cell devotes valuable resources to producing protein, other important functions may be neglected. Our work suggests that one driver of both abnormal and normal aging could be accelerated protein turnover.”
HGPS is a very rare genetic disease, causing people to age eight to ten times faster than normal and leading to premature death. The rare mutation occurs in one of the structural proteins in the cell nucleus—lamin A—but it has been unclear how a single defective protein in the nucleus causes the myriad rapid-aging features seen in the disease.
The Salk researchers were initially interested in whether the mutation was making the lamin A protein less stable and shorter lived. After measuring protein turnover in cultured cells from skin biopsies of both progeria sufferers and healthy people, they found that it wasn't just lamin A that was affected by the disease.
“We analyzed all the proteins of the nucleus, and instead of seeing rapid turnover in just mutant lamin A and maybe a few proteins associated with it, we saw a really broad shift in overall protein stability in the progeria cells,” noted lead study investigator Abigail Buchwalter, Ph.D., a staff scientist at the Salk Institute. “This indicated a change in protein metabolism that we hadn't expected.”
In addition to the rapid turnover of proteins, the researchers found that the nucleolus, which makes protein-assembling structures called ribosomes, was enlarged in the prematurely aging cells compared to healthy cells.
“…we report a widespread increase in protein turnover HGPS-derived cells compared to normal cells. We determine that global protein synthesis is elevated as a consequence of activated nucleoli and enhanced ribosome biogenesis in HGPS-derived fibroblasts,” the authors wrote. “Depleting normal lamin A or inducing mutant lamin A expression are each sufficient to drive nucleolar expansion. We further show that nucleolar size correlates with donor age in primary fibroblasts derived from healthy individuals and that ribosomal RNA production increases with age, indicating that nucleolar size and activity can serve as aging biomarkers.”
Intriguingly, the team found that nucleolus size increased with age in the healthy cells, suggesting that the size of the nucleolus could not only be a useful biomarker of aging but also potentially a target of therapies to counter both premature and normal aging.
“We always assume that aging is a linear process, but we don't know that for sure,” Dr. Hertzer concluded. “A biomarker such as this that tracks aging would be very useful, and could open up new ways of studying and understanding aging in humans.”