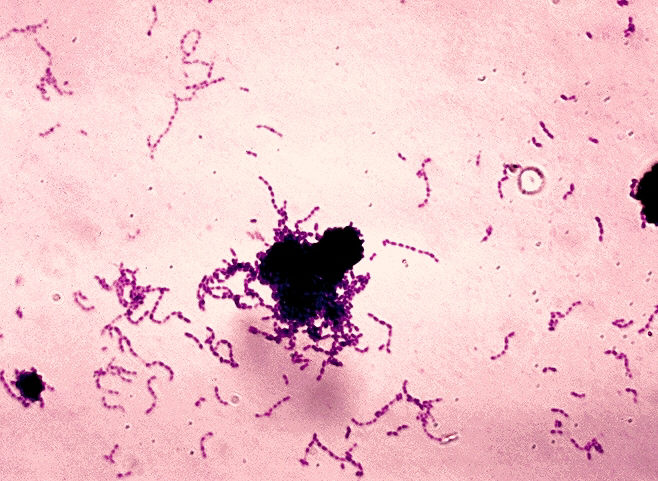
Tooth decay is bad enough when it is caused by Streptococcus mutans, a common bacterium that forms tartar. But tooth decay is even worse if S. mutans is joined by a relatively rare bacterium, S. sobrinus. When S. sobrinus is present, the sugars we eat are converted into acids more quickly, accelerating the decalcification of teeth and the formation of cavities.
To find out why S. mutans and S. sobrinus make such a destructive duo, a team of bioengineers at the University of Illinois College of Engineering sequenced the S. sobrinus genome. Evidently this work was a little like pulling teeth. S. sobrinus isn’t found in all people, and it is difficult to work with in the lab. Nonetheless, the bioengineers, led by Paul Jensen, Ph.D., assistant professor, University of Illinois College of Engineering, were able to sequence the complete genomes of three strains of S. sobrinus.
Details of this work appeared July 26 in the journal Microbial Resource Announcements, in an article titled, “Complete genomic sequences of Streptococcus sobrinus SL1 (ATCC 33478 = DSM 20742), NIDR 6715 (ATCC 27351 & 27352), and NCTC 10919 (ATCC 33402).” The article describes how genome sequencing was carried out using both Illumina short-read and Oxford Nanopore long-read technology. The article also makes the case that the genomic sequences obtained in the study represent “an important step toward understanding the genetic basis of cariogenesis by S. sobrinus.”
“We present complete circularized genome sequences for four strains of S. sobrinus, type strain SL1, strain NIDR 6715-7 and the related NIDR 6715-15, and strain NCTC 10919,” the article’s authors wrote. “The finished genomes will enable genomic comparisons between S. sobrinus and other cariogenic microbes.”
Now that the S. sobrinus sequencing is complete, Dr. Jensen and his students are building computational models to better understand how the two bacteria interact and why S. sobrinus can cause such potent tooth decay when combined with S. mutans.
Already they have confirmed, for example, that S. sobrinus lacks complete pathways for quorum sensing, which is the ability bacteria have to sense and react to nearby bacteria, and ultimately proliferate.
“Although it is rare, S. sobrinus produces acid more quickly and is associated with the poorest clinical outcomes, especially among children,” noted Dr. Jensen. “If S. sobrinus is present along with S. mutans, you're at risk for rampant tooth decay, which means there's some level of communication or synergy between the two that we don't understand yet.”
According to Dr. Jensen, S. mutans bacteria send out feelers in the form of a peptide to find out how many other S. mutans cells are nearby. Once the S. mutans cells reach a certain threshold, they attack and create an imbalance in a person's mouth between good and bad bacteria, which leads to rapid cavity formation.
“S. sobrinus doesn't have a complete system to do this,” noted Dr. Jensen. “We're really curious to explore this further and find out what is missing and why.”
The Illinois team has uploaded the sequencing information to the GenBank public database so scientists worldwide will have access to the S. sobrinus genomic information.