Helen Albert Writer GEN
Harnessing the Power of the Human Microbiome to Improve Health and Target Disease
We are all colonized by vast quantities of microorganisms. Until recently, there has been little focus on how these microbes can help us stay healthy. However, it has now been recognized that they can provide us with a whole array of different benefits. In contrast, disruption of these microbial communities, such as through excessive use of antibiotics, can actively contribute to disease.
The quest to try to understand the human microbiome and to discover how manipulating it in different ways could help prevent or treat disease has been taken up by the research community with enthusiasm, with advances in technology helping to power this search for knowledge.
There are several different microbiomes present in or on the human body such as that of the skin, the mouth and the urogenital tract, but the largest and most well characterized is the one found in the gut. Recent research has uncovered associations between the gut microbiome and a number of diseases including inflammatory bowel disease, diabetes, cancer, infections, and even neurologic disorders.
While a lot of this research is still at an early stage, clinicians and researchers are already finding ways to use their findings to help treat patients develop better diagnostic tools for disease; and evaluate the efficacy of different therapies based on the diversity and composition of a patient’s gut microbiome.
Conquering Clostridium difficile
An early win for gut microbiome researchers was the use of fecal transplants from healthy volunteers to treat patients with recurrent C. difficile infection—a condition that causes around 29,000 deaths per year in the United States alone.
The success of this somewhat unconventional treatment led to it cautiously being recommended for severe or reoccurring C. difficile infections by both the U.S. FDA and the U.K. National Institute for Health and Care Excellence. But, there are restrictions on its wider use due to concerns about transplant regulation and possible side effects (see “How Safe are Fecal Transplants” sidebar).
Mark Wilcox, M.D., professor, medical microbiology, University of Leeds, explained how the gut microbiome impacts on C. difficile infection: “The intact gut microbiome is hostile to C. difficile. When the gut microbiome is damaged, typically by antibiotics, this provides a niche which can be exploited by C. difficile.”
Wilcox and his colleagues advocate prevention as a way of tackling recurrent C. difficile infections. “By preventing the damage to the gut microbiome, we can potentially avoid C. difficile infection and the recurrences that typically occur in about a quarter of affected patients,” he said.
The research team has developed a laboratory gut model that allows accurate prediction of which antibiotics put patients at higher risk of developing C. difficile infection and which are most likely to be effective for treatment purposes.
The team also had success using targeted antibiotics to treat C. difficile infection. These include ridinilazole—a drug that appears to be better at preserving the gut microbiome and reducing the risk of recurrent infections than more traditional, less specific, antibiotics such as vancomycin.
Cracking Open the Cancer Microbiome
Jennifer Wargo, M.D., is associate professor of surgical oncology at University of Texas, MD Anderson Cancer Center in Houston. She first became interested in the influence that bacteria have on cancer while working at Harvard University.
“We identified bacteria within tumors that could mediate therapeutic resistance. Specifically, in 75% of patients with pancreatic cancer you could identify bacteria within their tumors and these bacteria could actually break down chemotherapy,” she explained.
After moving to MD Anderson in 2013, Wargo and her group began to focus more on how differences in the gut microbiome could impact on cancer treatment. Working with her colleague Vancheswaran Gopalakrishnan, Ph.D., also based at MD Anderson, Wargo and her team collected oral and gut microbiome samples from more than 200 patients with metastatic melanoma both before and after treatment with anti-PD1 based immunotherapy.
“Patients who had a higher diversity of bacteria within their gut had a better response to therapy than those who did not,” explained Wargo. Adding that “component taxa also mattered.”

Metastatic Melanoma Cells: The ability of cancer cells to move and spread depends on actin-rich core structures such as the podosomes (yellow) shown here in melanoma cells. Cell nuclei (blue), actin (red), and an actin regulator (green) are also shown. [Julio C. Valencia, NCI Center for Cancer Research]
Gopalakrishnan continued: “Taking our findings in our human cohort forward, we understood that the data was compelling, but it was mostly correlative and so we wanted to delve into the mechanism a little more. With that in mind we planned and performed experiments in germ-free mice.”
The researchers transplanted stool samples from human responders and non-responders to therapy into the mice. The mice were injected with cancer cells and subsequently treated. “Strikingly, we saw that the mice that were transplanted with a responder microbiome did much better on therapy than those with a non-responder microbiome,” said Gopalakrishnan.
Wargo and Gopalakrishnan hope to conduct human clinical trials of cancer patients based on their findings in mice. While currently the planning stages, the team hopes the first trials will start before the end of 2018.
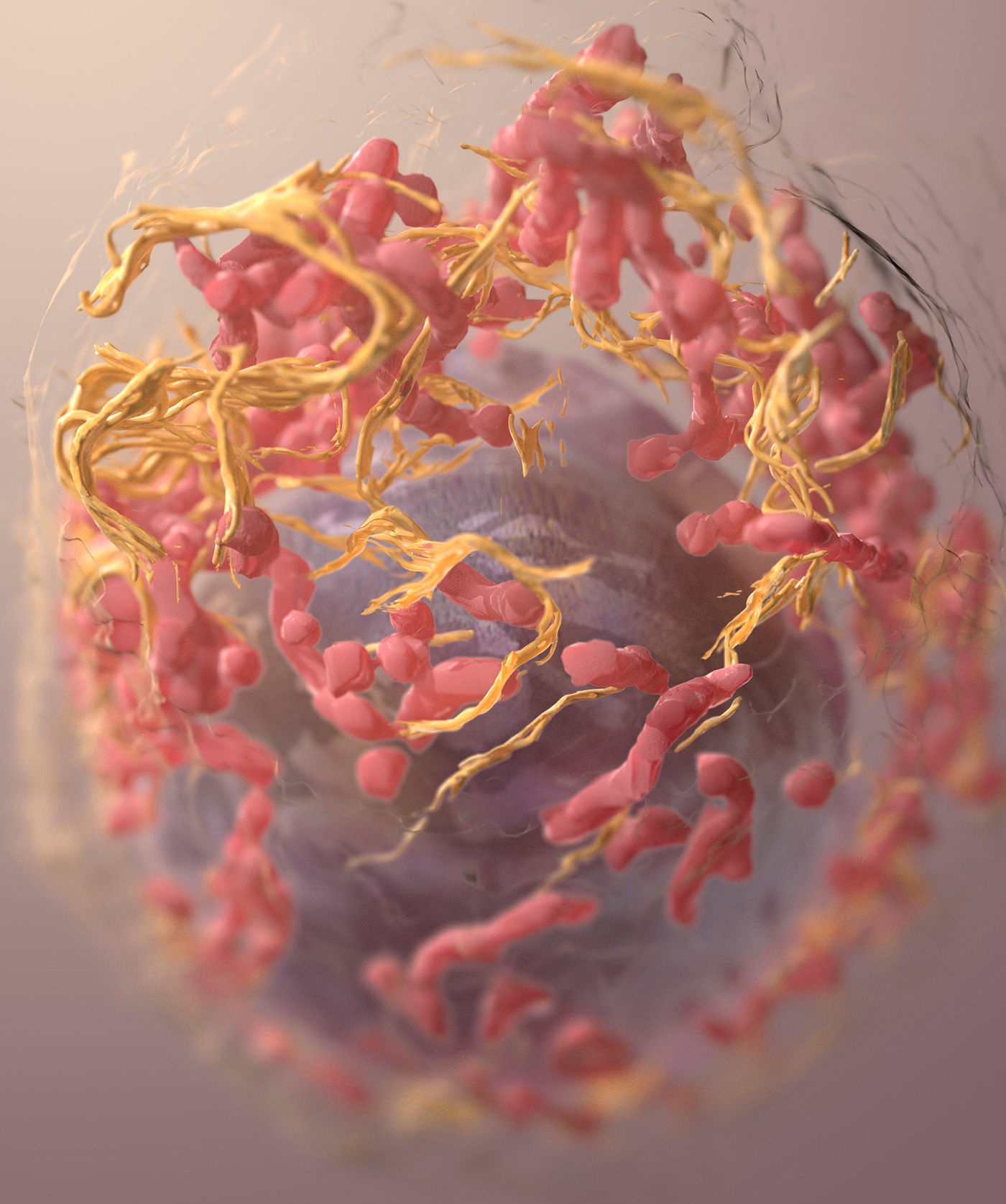
3D structure of a melanoma cell derived by ion abrasion scanning electron microscopy. [Sriram Subramaniam / NCI, NIH]
Implicated in MS
Imbalances in the gut microbiome have also been linked to a number of other conditions including multiple sclerosis (MS).
Ashutosh Mangalam, Ph.D., from the University of Iowa, and his team are one of several groups that have observed such imbalances in MS patients. He explained: “Based on the published MS microbiome studies we can say that patients have higher abundance of gut bacteria with proinflammatory characteristics and a lower abundance of gut bacteria with anti-inflammatory capabilities.”
In a recent study, Mangalam and his colleagues showed that the bacteria Prevotella histicola could suppress disease in a mouse model of MS. These findings are supported by reports from other groups that MS patients have a lower abundance of Prevotella species compared to people without the condition.
“Based on these findings we can hypothesize that bacteria belonging to the Prevotella genus are anti-inflammatory and might be used in future as a potential treatment option for MS patients,” suggested Mangalam.

University of Iowa’s Ashutosh Mangalam, Ph.D., with a colleague.
Simplicity is Key
While a large portion of the research being carried out in this area focuses on the gut microbiome, a number of researchers are investigating microbial communities found in other parts of the body.
Gregory Buck, Ph.D., and colleagues from Virginia Commonwealth University are researching the vaginal microbiome and how it affects women’s health, particularly during pregnancy.
In contrast to the gut microbiome, simplicity is key for the vaginal microbiome. “A homogeneous vaginal microbiome has traditionally been considered healthy, usually one consisting primarily of Lactobacillus species,” Buck explained.
He and his team are working on a longitudinal study that is part of the larger NIH-funded Human Microbiome Project. The Multi Omic Microbiome Study-–Pregnancy Initiative is looking at changes in a number of different microbiomes in pregnancy.
“There is evidence that adverse outcomes in pregnancy, including preterm birth, are influenced by the microbiome of the female reproductive tract,” said Buck. “We think adverse outcomes are loosely associated with having a complex microbiome, which is the microbiome type that would traditionally be associated with bacterial vaginosis. So some of the usual suspects for urogenital health are also usual suspects for adverse outcomes in pregnancy.”
Future Directions
While research into the microbiome is picking up steam, these efforts have only produced limited improvements in patient care to date.
Fecal transplantation has improved the lives of many patients with recurrent C. difficile infection, and will no doubt continue to do so. It’s thought it could also serve as a treatment for other conditions, but research is still at an early stage. For example, there have been four randomized controlled trials published using fecal transplants to treat ulcerative colitis and three of them showed significant promise.
“Targeted, microbiome-based therapies could hopefully remove some of the possible risks associated with fecal microbiota transplantation,” suggested Wilcox, “particularly given the potential far reaching consequences of manipulating this powerful ‘organ’ within us.”
When asked about the future, Wargo emphasized the importance of diet. “We know that the diet and microbiome are tightly related, and so I think with research in that area we are going to be able to counsel patients much better to have a diet that facilitates a more favorable microbiome.”
Mangalam and his team are also interested in the impact of diet. “We are investigating how gut bacteria might affect the host physiology through metabolism of common food. Our pilot studies suggest that small metabolites from phytoestrogens and bile acid metabolism can regulate immune system development,” he said.
The view of researchers seems to be that while it is now common knowledge the microbiome plays a critical role in human health, more in-depth understanding about the mechanisms behind the observed effects is needed for the development of effective new treatments for disease.
“This is undoubtedly the era of the microbiome,” said Wilcox. “I believe that we’ll soon be able to target therapeutics more effectively via an improved understanding of their desirable and unwanted effects on patients’ microbiomes.”
McIlroy concluded: “Moving from correlation to causality will allow us to develop precision microbiota-based diagnostic and therapeutic tools for currently unmet clinical needs.”

Visualization and localization of commensal bacteria by Fluorescence in situ Hybridization (FISH) in the small intestine of Toxoplasma gondii-infected mice. [NIAID, NIH]
This article was originally published in the November/December 2017 issue of Clinical OMICs. For more content like this and details on how to get a free subscription, go to www.clinicalomics.com.



