Patricia F. Fitzpatrick Dimond Ph.D. Technical Editor of Clinical OMICs President of BioInsight Communications
With the last shuttle taking off on July 8, scientists say they won’t have as many opportunities to conduct life sci research.
Some talking heads keep saying that the U.S. got very little return on its investment in costly and sometimes disastrous space shuttle missions. Life scientists with experiments aboard the shuttles would argue that point, as many of these studies were impossible to perform in earth’s gravity and have yielded remarkable information.
When the space shuttle Atlantis lifted off from Kennedy Space Center on July 8 on its last mission, it carried biological experiments that scientists hope will increase knowledge into how plants, animals, and humans sense and respond to space environment including microgravity. This shuttle mission, designated STS-135, also provided one last unique opportunity to test novel therapeutics that could work for ailments of earth-bound life, like osteoporosis.
In microgravity bone loss occurs at 1 to 1.5 percent a month. This leads to an acceleration of age-related changes like osteoporosis. Muscle mass loss also occurs as well as diminished cardiac function among other conditions.
Multiple collaborative efforts among governments, private, and academic enterprises have devised ingenious experiments to test the effects of space on cultured cells, plants, and animals, as well as the effects of novel therapeutics in these models.
Regenerative Processes in Microgravity
The Space Tissue Loss program, a collaboration between NASA and the Department of Defense (DoD), sent 20 sets of experiments into space on 18 shuttle launches since March 1992. It is part of the DoD Space Test Program (STP), which uses cell and tissue cultures in microgravity to study its effects on tissue regeneration and wound healing in space.
Eduardo Almeida, Ph.D., the stem cell regeneration program principal investigator and a scientist at NASA’s Ames Research Center, pointed out to GEN that gravity is important for stem cell health. “In its absence, tissue atrophy occurs, and repair does not occur normally.” He described two experiments aboard space shuttles Atlantis and Discovery.
In one experiment he and his colleagues used a model of stem cell development: mouse embryoid bodies that on earth form a sphere containing patches of differentiated cells such as neurons and muscle cells. “Everything went okay on the ground,” he said of the earth-bound controls, “but failed almost completely in space, as the embryoid body cells did not differentiate, failing to express normally 45 out of 52 differentiated tissue markers from mesoderm, endoderm, and ectoderm lineages, and continued to express stem cell markers such as Sox1 and Sox2.”
In another experiment Dr. Almeida’s team tested the ability of keratinocytes (differentiated from stem cells in space) to migrate correctly. Keratinocytes are key cells that participate in wound closure and healing. In space the cells lost nearly half of their migratory capacity, suggesting one cause of lack of efficient wound healing in microgravity.
“For mammalian cells, microgravity is not replicable on the ground,” Dr. Almeida explained. “The only way to do these experiments is to go up.” The inability to do experiments in space “is frustrating because it’s really inefficient to do space biology experiments on earth with inadequate experimental models. A year of NASA spaceflight research is worth 10 years of ground research because of the wealth of unique insights space provides.”
For space travel, he said, the current research highlights the importance of gravity in promoting stem cell regenerative health. “For medicine on earth, this space research makes clear that forces generated by gravity are required to promote normal tissue regenerative processes and teaches us that sometimes we need to step back from things like gravity that we take for granted to understand their fundamental importance for life on earth.”
Tissue Genesis (TGI) has provided science experiments, hardware/payload integration, and adult stem cells in support of the NASA/DoD research collaboration. Tissue Genesis vp and GM, Thomas F. Cannon, told GEN that the company’s core expertise is in space shuttle cell experimentation.
“We were the science/hardware integrators for three experiments flown on the STS-135 DoD Space Test Program manifested Cell Culture Module (CCM),” he said. The CCM is a hollow fiber-based automated perfusion cell culture payload, originally developed at the Walter Reed Army Institute of Research, Cannon explained. It performs a variety of automated injections, collections, and culture manipulations.
Tissue Genesis also participated in a Telemedicine and Advanced Technology Research Center (TATRC), USAMRMC-sponsored experiment that cultured and grew adipose-derived regenerative cells recovered by the TGI Cell Isolation System.
Back at TGI, Joon Paek, Ph.D., the project PI, evaluated the trophic factors and immunomodulatory response of the cells in the space environment. “We have the cells now back in our lab and they are continuing to pump out growth factors. Hopefully we will have some exciting results to report. The two other investigators—Dr. Almeida, and Dr. Rasha Hammamieh, USAMRMC—are reporting very good preliminary results as well.”
Bone and Muscle Loss
Since 2001, Amgen has also been conducting tests in space. Its research has explored three compounds developed to combat bone or muscle loss. Amgen treated mice with osteoprotegerin (OPG) to determine whether the molecule could prevent bone loss when the animals were in zero gravity conditions. Results of the OPG experiment showed that a single, preflight treatment of the mice with Amgen’s OPG prevented bone loss.
OPG, a secreted glycoprotein of the tumor necrosis factor (TNF) receptor superfamily, inhibits bone resorption by preventing osteoclast maturation. Amgen scientists developed a recombinant version of the protein. The experiment was conducted on NASA’s space shuttle Endeavour (STS-108), launched from the Kennedy Space Center in November 2001 and coordinated through BioServe Space Technologies, a NASA-sponsored Research Partnership Center (RPC) located at the University of Colorado in Boulder.
In 2007, on board NASA’s shuttle flight STS-118, flown by the orbiter Endeavour, Amgen tested an experimental myostatin inhibitor for its effectiveness in preventing space flight-induced muscle changes in mice. Also coordinated through BioServe, this was the first time an experimental therapeutic for muscle loss was investigated in space, according to Amgen.
The company is developing a myostatin inhibitor known as AMG 745 for muscle wasting disorders. The compound is currently in Phase I studies and is distinct from the molecule used in the 2007 space studies.
Additionally, this July Amgen and partner UCB tested their sclerostin antibody on board the shuttle Atlantis during its last mission. It is designed to inhibit the action of sclerostin, a protein that negatively regulates bone formation, bone mass, and bone strength. Thirty mice were flown in space, with half of the rodents given a preflight injection of the sclerostin antibody and the remaining mice given placebo.
Now, after the flight has landed following 13 days in space, various aspects of the structure, composition, strength, as well as cellular and molecular nature of the bones from the flight- and ground-based control mice are being analyzed. The findings, the company has said, may provide insights into treating the skeletal fragility resulting from “skeletal disuse” in such conditions as immobilization, stroke, cerebral palsy, and spinal cord injury.
The space shuttle experiments were important for gaining further insight into this new bone-forming pathway, Amgen’s scientific executive director, Chris Paszty, Ph.D., told GEN. “A one-way trip to Mars takes 7 to 9 months, then astronauts spend about a year working there, then take another 7 to 9 month trip back,” he said.
“For such extended-term space travel, having agents that can prevent bone loss and/or can actively build new bone would be very important. Given that the sclerostin pathway is regulated by mechanical loading, we were thrilled to have this opportunity to test our sclerostin antibody under complete unloading, in the zero gravity environment of outer space,” Dr. Paszty commented. Amgen’s hope, he added, is that a similar antibody will one day provide an option for treating bone-related disorders and diseases.
AMG 785/CDP7851, a different sclerostin antibody than the one being used for the STS-135 space study in mice, is currently in Phase II trials for bone-related conditions under Amgen’s agreement with UCB. Both antibodies bind to and inhibit sclerostin, but the one being used in the space shuttle study is a mouse antibody.
In April, Amgen and UCB reported positive results from the Phase II study comparing AMG 785/CDP7851 to placebo in postmenopausal women with low bone mineral density for the treatment of postmenopausal osteoporosis. The study met its primary endpoint, showing increases in lumbar spine bone mineral density at month 12 for the AMG 785/CDP7851 active arms versus the placebo arm. In addition, the drug compared positively with the two active comparators, teriparatide and alendronate.
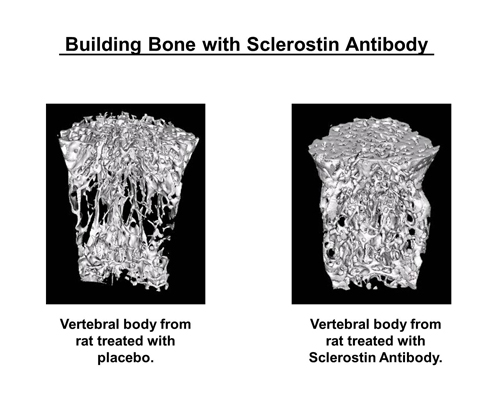
Images show the vertebrae of a rat with bone loss after treatment with placebo and another rat with bone loss after treatment with the same Amgen antibody that was injected in the mice sent into space last month aboard the last Atlantis mission.
Changing Protocols for Research
Commenting on the loss of the shuttle missions, Louis Stodieck, director of BioServe Space Technologies, said in Nature that neither the option of using commercial space cargo services such as Space Exploration Technologies’ Dragon or Orbital Sciences’ Cygnus spacecrafts offers a “great option for returning samples.”
So, he added, scientists will have to adapt their research protocols. “Because we will have less ability to bring things back, we will be doing analyses in space and sending the data down, not the samples,” he pointed out.
For scientists who have devoted considerable time and effort devising experiments that take advantage of rocket science to gain insights into biological science, few shots remain for getting studies into space.
Patricia F. Dimond, Ph.D. ([email protected]), is a principal at BioInsight Consulting.