
A century after researchers from the University of Toronto discovered the hormone as a treatment for diabetes in humans, insulin—and recombinant DNA versions brought to market in the past four decades—remain the most common means of controlling blood sugar for people with the disease. However, researchers and companies focused on diabetes have long promoted the possibility of treating the disease through alternative approaches that include cell therapies.
Among companies focused on cell therapies for diabetes is San Diego-based ViaCyte, which says that its portfolio of stem-cell derived therapies includes first-in-category, first-in-class products that represent major advances toward a functional cure for type 1 diabetes (T1D) and other chronic diseases.
Funding the development of functional cure therapies for T1D is the intended use of proceeds for ViaCyte’s latest financing, which it closed in June. ViaCyte closed the Series D with a final tranche of $45 million, bringing the total raised in this round to more than $115 million.
New investors Adage Capital Partners, Invus Group, Asymmetry Ventures, and Artis Ventures joined existing investors Bain Capital Life Sciences, TPG Capital, RA Capital Management, Sanderling Ventures, and undisclosed “long-time insiders” in the Series D, which accounts for just over half of the $225 million in total capital raised by ViaCyte.
“We have a pluripotent stem cell line that behaves very well and provides an unlimited source of cells. The idea is, scientifically through our proprietary technology, we can differentiate that across the various cell types and, eventually, they differentiate and mature into pancreatic beta cells to produce insulin,” ViaCyte president and CEO Michael J. Yang told GEN Edge.
‘We’re very excited about some of the data that we’re generating, have generated, and will generate,” Yang added. The goal is “to eventually get to a world where we would functionally cure type 1 diabetes, disintermediating the need for insulin, having a set of cells that physiologically act like a pancreas for the patient.”
Cell-Based Scramble
ViaCyte is among companies competing with biopharma giants as well as smaller biotechs to develop cell-based diabetes therapies.
Furthest along among competitors is Vertex Pharmaceuticals, which entered the field in 2019 when it acquired Semma Therapeutics for $950 million cash. In March, Vertex began a Phase I trial (NCT04786262) of VX-880 in T1D patients with severe hypoglycemia and impaired hypoglycemic awareness. The study’s estimated primary completion date is January 2024.
Vertex’s trial of VX-880 followed research by the lab of Doug Melton, PhD, Co-Director of the Harvard Stem Cell Institute, that began in 2000 with the goal of creating pancreatic beta cells from stem cells—something his lab achieved in 2014 and recognized among the top discoveries of 2014 by Science. The FDA has granted VX-880 its Fast Track designation.
Subsequently, Semma successfully showed it could produce large quantities of functional human beta cells that restore insulin secretion and ameliorate hypoglycemia in animal models, as well as a novel device designed to encapsulate and protect the cells from the immune system, enabling durable implantation without the need for ongoing immunosuppressive therapy.
Also developing a cell therapy for T1D are Eli Lilly and Sigilon Therapeutics. The companies are in IND-enabling studies for SIG-002, an islet cell replacement product candidate based on Sigilon’s Shielded Living Therapeutics™ platform for producing non-viral engineered cell-based therapies. The platform is being applied in Sigilon’s lead program, a cell therapy for Hemophilia A that is expected to read out data later this year.
“If the platform starts to show positive data in Q3, it could bounce back and de-risk the technology and increase the value of the platform and the pipeline,” Michael Yee, analyst with Jefferies, wrote in May.
Sigilon has included SIG-002 among candidates for which it said it could submit IND filings by the end of 2022, according to the company’s Form 10-K annual report for 2020, filed March 18.
Lilly agreed to partner with Sigilon in 2018, in return for paying the Cambridge, MA, biotech $62.5 million upfront, and a $13.1 million equity investment. Lilly has also agreed to pay Sigilon up to $165 million tied to achieving regulatory milestones, $250 million tied to sales-based milestones, and tiered mid-single-to-low-double digit sales-based royalties on sales. Lilly invested an additional $12 million as part of Sigilon’s Series B financing, completed in 2019.
Creating a market
Lilly created a market in 1923 when it commercialized the insulin discovered in Toronto by Sir Frederick Grant Banting and Charles Best two years earlier, launching Iletin® as the world’s first commercially available insulin product for diabetes control in humans, using material harvested from cows and pigs.
Nearly 60 years later, in 1982, Lilly successfully developed a human source of insulin safe and effective enough to supplant animal-derived versions, winning FDA approval for Humulin® as the first marketed human healthcare product derived from recombinant DNA (rDNA) technology.
Another successful developer of recombinant DNA insulin, Novo Nordisk, has invested in human stem cell technology since 2008, working to generate a protocol for stem cell-derived insulin producing islet-like clusters. Novo Nordisk told analysts earlier this year that it plans to file an IND in the coming years for a preclinical cell therapy candidate to be developed for T1D—after it is first developed for Parkinson’s disease.

“We hope to celebrate that at the 100-year anniversary—not of insulin in Toronto but of the Novo Nordisk company” [in 2023], Novo Nordisk’s Mads Krogsgaard Thomsen quipped in February during the company’s full-year 2020 earnings call, shortly before retiring as the company’s Executive Vice President and Head of R&D.
ViaCyte was created in 2004 following a three-way merger of Novocell, based in Irvine, CA; Cythera, based in San Diego; and BresaGen, based in Athens, GA. In 2010, the combined company changed its name from Novocell to ViaCyte, with the aim of reinforcing its identity as a leading cell therapy developer, as “-cyte” is derived from the Greek word “kytos” or cell.
Today, ViaCyte’s pipeline includes three cell-based approaches to fighting diabetes. The pipeline is co-led by two Phase II candidates, both islet cell replacement therapies that deploy PEC-01™ pancreatic progenitor cells made from the pluripotent stem cell line CyT49. Once implanted and successfully engrafted, the cells mature into beta cells that secrete insulin, alpha cells that secrete glucagon, and other cells of the human pancreas that naturally control blood glucose levels.
“Like a normal pancreas”
“The cells that we put into the devices act like a normal pancreas,” Yang explained. “So ‘functional’ means that they produce insulin, they produce glucagon, and the patients eventually won’t have to take exogenous insulin. They can live their lives normally.”
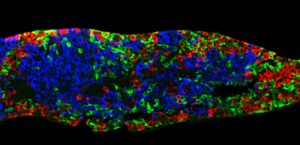
On June 25 at the American Diabetes Association’s Virtual 81st Scientific Sessions, ViaCyte presented preliminary data from one patient in the Phase I/II VC02-101 trial (NCT03163511) showing PEC-Direct to have effectively engrafted and produced C-peptide, a biomarker used to assess production of insulin by functional pancreatic beta cells. ViaCyte said the data represented the first exhibition of implanted pancreatic progenitor cells producing endogenous insulin in a patient.
Nine months following implantation with PEC-Direct, the patient showed three clinically relevant measures: Increased glucose-responsive C-peptide levels, with other data indicating that the cells were producing and secreting insulin; and two results that suggested improved control of blood glucose with the therapy, increased time in range, and decreased HbA1C. The results also showed improvement from the week 26 data reported in an abstract (presented at the ADA event.)
Significant step
“The ability of the PEC-Direct cell therapy to achieve clinically relevant C‑peptide levels accompanied by an increased time in range is a significant step in the development of a cure for patients with T1D,” said the trial’s Principal Investigator, Bart Keymeulen, MD, PhD, of University Hospital Brussels. The study is enrolling additional patients toward an estimated enrollment of 75 and is expected to read out additional data in a subset of additional patients in the first half of 2022.
Enrollment was halted last year by the COVID-19 pandemic: “We have plenty of patients, and the sites are primed. We were just waiting for COVID protocols and vaccines,” Yang said.
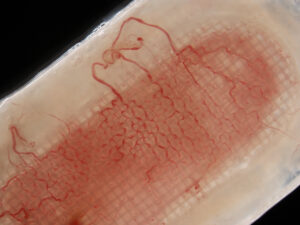
In addition to improving engraftment, the encapsulation system is also designed to enhance cell survival and overall function by reducing foreign body response and protecting the cells from the patient’s immune system, thus eliminating the need for immune suppression drugs used with other transplants.
ViaCyte has received funding and research support from the California Institute for Regenerative Medicine (CIRM) and JDRF—which have awarded the company $72.3 million and $13.6 million, respectively, in grants toward R&D of its technologies and product candidates.
ViaCyte and Gore expanded their collaboration in March beyond PEC-Encap, by extending research and clinical development across ViaCyte’s full product portfolio and Gore’s full materials and device proprietary technologies.
In February, ViaCyte and Gore began implanting the first patients in the Phase II VC01-103 trial (NCT04678557), designed to assess the safety, engraftment, and efficacy of PEC-Encap after a year in patients with T1D. ViaCyte said it plans to enroll an initial cohort of 10 patients in the open-label study, with the potential to expand enrollment to up to 70 patients.
The study is expected to release safety and efficacy data later this year.
CRISPR collaboration
ViaCyte’s third candidate, PEC-QT™ (VCTX210), engineers the company’s CyT49 pluripotent human stem cell line via gene editing to avoid destruction by the patient’s immune system, potentially eliminating the need for immunosuppressants. The cell line will be differentiated into pancreatic endoderm cells, which will likely be housed in the same type of device used for PEC-Direct, allowing for direct interaction between blood vessels and implanted cells.

PEC-QT is being developed through a collaboration with CRISPR Therapeutics that was launched in 2018 and generated $15 million in upfront cash for ViaCyte, which has the option under certain circumstances to receive an additional $10 million from CRISPR Therapeutics in the form of a convertible promissory note.
ViaCyte plans to launch clinical studies of PEC-QT by the end of this year, Yang said.
“The ability to gene edit cells to make them immune-evasive was very attractive to the propositions of driving towards the broadest patient population, when we talk about that functional cure,” Yang said. “The partnership we have with CRISPR Therapeutics is at the genetic level—they do the gene editing, we build the cells and differentiate them using our technology.”
Yang took ViaCyte’s helm in January, succeeding Paul Laikind, PhD, who retired after eight years in the position. Yang joined ViaCyte from Acadia Pharmaceuticals, where he served as Executive Vice President and Chief Commercial Officer. Previously, Yang was President of Johnson & Johnson’s Janssen Biotech, where he oversaw Janssen’s U.S. Immunology business, growing it into more than $8 billion in annual revenues.
Unrelated to Yang, Janssen in 2016 handed its BetaLogics cell-based diabetes development group to ViaCyte for an undisclosed price—two years after Janssen gave ViaCyte $20 million in return for a right of first negotiation to evaluate a transaction related to VC-01 (now PEC-Encap).
In addition to expanding its clinical presence with PEC-QT, ViaCyte also plans to grow its workforce beyond its current approximately 100 people, though the company is not disclosing details.
ViaCyte does say that as its candidates advance through clinical trials, it will expand its manufacturing, which takes place in two facilities in San Diego, where the company is based.
The second site, a leased facility which opened last year, includes 6,000 square feet of clean room space to meet needs through Phase III studies.
“We’re looking at process development improvements to move from roller bottles to bioreactors, and to improve our scale,” said Brittany K. Bradrick, ViaCyte’s Chief Financial Officer and Chief Operating Officer. “In this novel area of stem cells, where we’re differentiating our own stem cell line, we want to ensure the purity and the quality of that line. It’s very important for us to maintain the manufacturing in house, at least at this point.”


