
Genomics and synthetic biology pioneer George Church, PhD, says GRO Biosciences (GRObio), a developer of enhanced protein therapeutics he co-founded based on platform technology discovered in his Harvard Medical School lab, reflects a truism about startup formation.
“Every postdoc has an invention, but not every invention is something that we want to immediately launch,” Church, who heads GRObio’s scientific advisory board, observed recently on “Close to the Edge”, GEN Edge’s video interview series. “We try to incubate them as long as possible inside the lab until we’re sure that they’re mature enough so that we won’t get diluted out immediately by running out of VC [venture capital] money.”
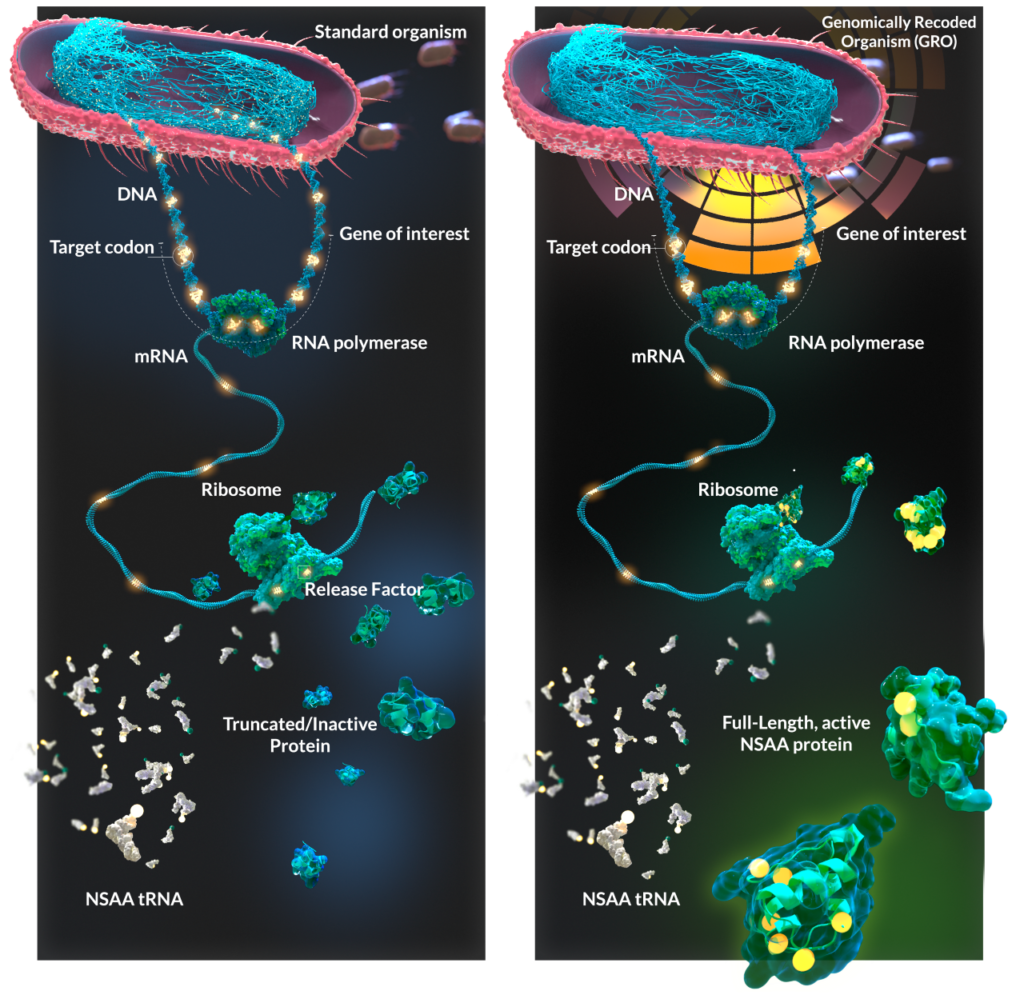
GRO stands for “genomically recoded organisms”—the first production organisms made with modified genomes and engineered protein translational machinery, according to the company.
By recoding the genomes of bacterial strains used in biologics production, GRObio reasons that it can expand beyond the 20 amino-acid building blocks typically found in proteins to introduce non-standard amino acids (NSAAs) that can customize the shape and chemical properties of the protein.
“What we do is to systematically alter the genetic code,” Daniel J. Mandell, PhD, who is GRObio’s CEO, summed up to GEN Edge.
Church’s Harvard Medical School lab first described and characterized GROs in a paper published in 2013 in Science. In 2015, Church, Mandell and seven co-authors reported in Nature their successful computational redesign of essential enzymes in the first organism possessing an altered genetic code, conferring metabolic dependence on NSAAs for survival.
Also in 2016, Church, Mandell, and four others co-founded GRObio to commercialize the technology by developing protein therapeutics based on computational protein design and synthetic biology technologies. Among the co-founders are Andrew Ellington, PhD, whose lab at the University of Texas at Austin focuses on developing novel genetic codes and synthetic organisms based on engineering the translation apparatus; and Christopher Gregg, PhD, GRObio’s chief scientific officer (CSO).
Mandell was wrapping up a PhD in computational protein design at University of California, San Francisco, about a decade ago, and seeking a postdoctoral position when he came across Church’s research on GROs.
“It was an epiphany”
“For me it was an epiphany: Now we can go beyond the 20 amino acids and build designer proteins that can carry out almost any function. I came to George’s lab to bring these two worlds together of computational protein design and genome-wide recoding,” Mandell recalled.

“We did some early work demonstrating that you can in fact predictably engineer proteins whose folding and function depend upon non-standard amino acids, to convince ourselves that we really can do this in a rational way. That’s when we turned our attention to the question of what are the outstanding problems in the clinic that we might address using that technology.”
Mandell joined Church’s lab within a year of Gregg joining: “We very quickly realized we both had entrepreneurial designs and mesh very well, and we put a lot of time into thinking about how we could try to commercialize this technology.”
Gregg, now GRObio’s CSO, was pursuing a PhD in glycobiology, studying how glycans interact with the immune system. He started a short-lived synthetic biology startup focused on biofuel, before switching gears to integrating synthetic biology with organism and genome design.
“Luckily, I was able to get George’s interest in my thesis, which had to do with glycobiology and human-specific disease preponderances,” Gregg recalled. “I came in as the organism [GRO] was getting finished. It was just such a ripe platform for trying new things and solving new problems. Then when Dan and I realized that we had the same interests, we just started running with it.”
That pursuit paid off for GRObio last month, when it completed a $25-million Series A financing co-led by Leaps by Bayer and Redmile Group. Redmile is a San Francisco venture and private equity investment firm. Leaps is the equity investment arm created by Bayer to establish new companies and invest in early-stage technologies with breakthrough potential to “fundamentally change the world for the better.”
Bayer came to invest in GRObio, Mandell said, through relationships he had with the pharma/consumer goods/agbio giant and contacts from GRObio’s network of investors. Among investors joining the financing were Digitalis Ventures and Innovation Endeavors. (The Series A brings total investment in GRObio to $31.2 million so far.)
Proceeds from the financing, GRObio said, will support development of its GRO platform, a scale-up of bioprocess manufacturing, preclinical validation studies, and IND-enabling studies for GRObio’s pipeline of NSAA protein therapeutics designed to treat autoimmune and metabolic diseases.
“These are just initial focus areas,” says Mandell. “Autoimmune disease and metabolic diseases are a really a small part right of this broader universe [of opportunities]. But there are specific indications in there that we’re focusing on.” The company isn’t yet disclosing those indications, except to say they will address unmet clinical needs.
Beyond the pipeline
Taking this universe of NSAA chemistries, Mandell and colleagues want to ask some big questions. “Which problems really can’t be solved in the clinic without this new expanded universe of chemistries at the amino acid level? Some of these problems have interesting-looking solutions coming down the clinical development pipeline,” Mandell said. “That’s not really where we want to play. We want to play in areas where we think we can solve Holy Grail challenges and there isn’t another way to go about this.”
GRObio has constructed a “biofoundry” consisting of proprietary computational protein design and robotics pipelines, with the aim of streamlining development of NSAA translational machinery and NSAA protein products. The biofoundry applies strain and genome engineering, automation, analytics, and protein design software to build uniquely scalable NSAA protein “factories” from trillions of candidates.
GRObio emerged from stealth mode in 2017, raising $2.1 million in seed funding led by Digitalis Ventures, with participation from Innovation Endeavors, the venture capital firm whose co-founders include Eric Schmidt, Google’s former CEO and later executive chairman of Google and its parent company, Alphabet Inc.
From three people when it started, GRObio has since grown its staff to 16 people. “We will double in size by 2023,” Mandell said.
GRObio hopes its alphabet-expanding approach will enable it to grow its own significant share of a protein therapeutics market that according to Market Research Future (MRFR) is expected to increase over the next six years at a compound annual growth rate of 6.86%–for a nearly 60% rise to about $290.69 billion in 2027 from $182.69 billion this year.
GROs are intended for high-efficiency, commercial-scale production of proteins with NSAAs. These NSAAs are intended to enhance protein therapies with capabilities such as unprecedented duration of action and more precise control of the immune system.
GRObio is building its pipeline by advancing its first two product families of NSAA platform chemistries: DuraLogic™ is designed to enable flatter pharmacodynamic profiles and relaxed dosing schedules, while ProGly™—short for “programmable glycosylation”—are designed to produce biologics that enable the immune system to treat autoimmune disease, or to eliminate immunogenic side effects of protein-based therapies.
DuraLogic integrates NSAAs to enhance and maintain the three-dimensional structure of proteins needed for therapeutic activity. “That means things like resistance to proteases that can degrade the therapeutic, resistance to reducing agents that can break bonds in the protein that will render it inactive or misfolded, and the potential in some cases to increase the thermostability of the protein, so it can either last longer in storage, or just have a longer in vivo half-life,” Mandell said.
ProGly consists of glycan-containing NSAAs designed to induce or inhibit an immune reaction. GRObio says its GRO platform enables precise placement of defined ProGly compositions on the protein surface needed to elicit immune response.
Re-educating the immune system
“You can actually retrain or re-educate the immune system to treat a particular protein as self or non-self by administering that protein with a particular glycan’s signature. You give it what’s called a tolerizing signature,” Mandell explained. “The body will remember that this is a self-protein, and over a short period of time can begin to actually reverse the autoimmune response to that protein.”
GRObio expresses that protein in its platform, decorated with ProGly NSAAs that take on the form of human-like glycans.
“By putting that into your body, your immune system begins to learn that this is a self-protein and it builds up an immune memory of that protein and stops attacking it,” Mandell said. “This is really a way that we can begin to reverse a number of different autoimmune diseases, by taking an antigen-specific approach.”
That approach, he added, contrasts with broadly immunosuppressive drugs that turn down auto immune response by knocking down a patient’s immune system—an approach Mandell said increases susceptibility to infection and cancer and also often causing metabolic disorders: “What we want to do is re-educate the immune system to treat the one protein or the small number of proteins that you’re reacting to as self-proteins for the first time.”
The genetic code of each gene combines the four natural nucleotides of DNA—adenine (A), cytosine (C), guanine (G), and thymine (T)—to spell out three-letter codons specifying an individual amino acid. There are 64 different naturally occurring codons that encode 20 natural amino acids.
“What we did was to modify the genome to remove all the instances of one or more of those codons. Then, by installing on what we call new translational machinery from the cell that recognizes a particular codon, we can actually now reassign the meaning of part of the genetic code to direct the incorporation of a new amino acid,” Mandell said.
The company’s GRO platform expands the amino-acid alphabet to overcome limitations of protein therapeutics to address product stability, immunogenicity, and delivery into the cell.
Exploiting redundancies
“We’re exploiting redundancies in the genetic code to reassign redundant codons to new, non-standard amino acids, because there’s more than one way to specify a particular amino acid or stop,” Mandell explained.
For example, three codons code for the “stop” signal (UAG, UAA, and UGA), halting the elongation of a growing peptide chain. GRObio developed a recoded strain of bacteria that replaces all UAG stop codons with UAA stop codons and reprogrammed the UAG codon to new amino acids such as selenocysteine, a naturally occurring amino acid found in the cell, yet rarely incorporated into proteins.
By replacing all codons that code for cysteine residues in a protein with UAG, selenocysteine can be selectively incorporated in place of cysteine residues to form stabilizing diselenide bonds. Proteins stabilized with diselenides maintain stability and resist the reduction found both in human blood plasma, which destabilizes therapeutics, as well as in solvents and buffers, which destabilize industrial enzymes.
“It was quite evident that people were excited about working with the first recoded strain and they started looking around for what the best products were,” Church recalled. “The first thing we had published on the first NSAA we’ve published for biocontainment was bipA [biphenylalanine], and that did not seem like a good starting product. But diselenides, which we did in collaboration with Andy Ellington’s lab, looked like a great initial product, and that was quite enough to get the company launched.”
Gregg cited insulin as an example of a treatment that could be engineered for less frequent dosing through a selenocysteine NSAA that could be designed to prevent the breaking of bonds between the peptide chains that renders the molecule non-functional.
“Now all of a sudden,” he said, “you’ve got a fundamental mechanism by which you can say, this NSAA will support the survival of this molecule in this environment, on a timescale that we think will be beneficial to patients as a product.”
