October 1, 2007 (Vol. 27, No. 17)
Bruce Jon Compton Ph.D.
Jacob Peter Jensen
New Modes Are Beginning to Gain Ground on Fed-batch Strategy
The therapeutic success of highly efficacious complex biologicals that are almost exclusively produced in cell culture such as antibodies and fusion proteins means the biotechnology industry is now looking to improve the efficiency of production to increase output and to satisfy market demands. It has also led biopharmaceutical production and development teams to focus on decreasing the production costs or cost of goods (COG) of cell culture-based products.
Historically, the low titers and high cost of capital equipment, installation, qualification, and operations have meant that upstream production has dominated the COG’s calculation. However, continuous progress has been made in increasing product titers, which has had a direct impact on production costs. Currently one of the topics of interest in this field is the difference between the two main methodologies used today in the production of biopharmaceuticals from mammalian cell cultures, fed batch and perfusion.
Fed-batch Strategy
In fed-batch mode, media and other nutrients are added at fixed intervals to the culture, and the product is only harvested at the end of the run. This methodology is the most prevalent in the industry today; it is well understood and characterized, and produces higher yields than batch modes that do not involve feeds. Fed-batch systems are generally reliable; nutrients can be monitored and controlled, and media is used efficiently. As there is only one harvest, this leads to efficient process characterization, harvest of raw materials, and downstream processing.
There are some potential disadvantages to the fed-batch method. Waste products such as lactate and NH4+ accumulate and the culture degenerates during the run. This can lead to the production of proteases and other degradation enzymes, which can act on the desired product. Also, key nutrients may become exhausted. Since the conditions within the culture may be constantly changing, cells go through several phases of growth, peaks, and troughs of production, and product may be degraded prior to harvest.
The actual mean residence time of product in the reactor, which is kept at 37°C or lower, can be extensive and the actual distribution around this mean can span many days. Thus, product stability and heterogeneity can be an issue, however, given a stable product (or at least one that transforms to a desirable end point) this mode of production can be useful. In general, if a product has the profile of being stable with modest market needs (low kgs per year), fed batch is still the industry’s preferred production mode.
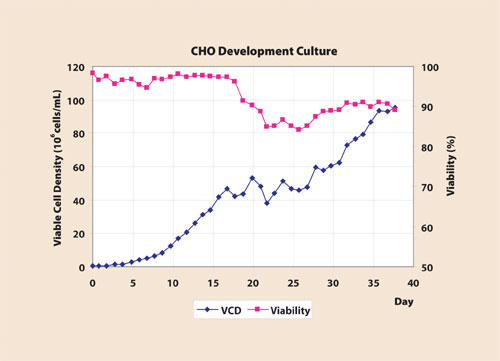
Figure 1
Perfusion Methods
In perfusion mode, media and other nutrients are continuously exchanged and product is harvested throughout the culture period. Thus, the process operates as a chemostat with cell retention, where the constant addition of fresh medium and the elimination of waste products provides the cells with the stable environment they require. This enables much higher cell densities to be achieved and, in turn, leads to higher productivity.
Once the culture has reached a steady state, the culture may be maintained for up to 100 days. Since product is harvested as it is produced, the mean residence time of the product in the reactor is low and degradation is less likely. Indeed, daily output from a perfusion system run with perfusion rates of up to two reactor volumes per day can be equal or even greater than the output from an entire fed batch. Thus, perfusion technology is highly suited to products that show toxicity or other adverse effects toward host cells and to unstable products (e.g., sensitive to proteolytic degradation, desialyation, deamidation, or low solubility at production pH).
Capital and start-up costs are lower for perfusion technologies, as smaller upstream and downstream capacity is required, and the process uses smaller volumes and fewer seed steps than batch methods. Perfusion is also more amenable to development, scale-up, optimization, parameter sensitivity studies, and validation.
The cost of batch failure due to contamination is reduced in perfusion technologies. Should contamination occur, product harvested prior to contamination will not be affected and can still be processed. If infection arises early in the run, then only a small volume of media will have been used, and if it arises later, significant product will have been harvested. In either scenario, the cost of failures is reduced when compared with fed batch, where generally the whole run will be lost.
Despite the potential benefits of the perfusion mode, fed batch has been the most widely used production methodology in recent years. In part, this is due to fed-batch culture being well characterized and in part due to the expertise within the industry. This is beginning to change however and there are now several examples of commercial production using perfusion including factor VIII (ReFacto®) and IgG (Remicade® and Simulect®).
There are several reasons why perfusion technology is on the increase:
•Perfusion-control technology and general support equipment is improving, and the overall process is increasingly robust.
•The technology allows for scalability and bioreactors of a size up to a working volume of 1,000 L can be used, dependent on process.
•Cell-retention systems and the scalability of these, which have always been the achilles heel of this mode of operation, are improving, and that leads to less cell loss and cell death and greater viable cell densities than seen previously.
•Higher cell density is being achieved with greater than 5×107 cells/mL being regularly achieved compared to 2×107 in fed-batch cultures.
•All of the operation improvements have led to low infection rates and reliable results.
All of these factors lead to higher product concentration in the harvest and better yields without significant increase in cost. Typically, cost of goods in the 1,000 L bioreactor scale compares with fed-batch cost in the 10,000–20,000 L.

Figure 2
Future Developments
The availability of a robust perfusion system based on a device such as the ATF Perfusion and Filtration System (Magellan Instruments;www.magellaninstruments.com), which allows for the use of a variety of membranes, opens the way for extensions to the basic operation mode.
Recent development results at CMC Biopharmaceuticals (www.cmcbio.com) have shown that the ATF allows for relatively high cell densities. Cell numbers up to 7×107–x108 cells/mL with viability >90% are achieved on a regular basis with CHO cell lines, with perfusion flow limited to less than two volumes/day (Figures 1 and 2). This, in combination with stable qP, gives higher total output of the protein of interest.
Another recent development is the XD™ high cell density perfusion process for PER.C6® from DSM (www.dsm.com). Through the use of ATF, it allowed generation of titers of IgGs up to 10 mg/mL, according to DSM. We anticipate this is just the beginning of the continued evolution of perfusion-based devices.
Bruce Jon Compton, Ph.D., is senior vp, business development, and Jacob Peter Jensen is manager, cell culture development, at CMC Biopharmaceuticals.
Web: www.cmcbio.com.
E-mail: [email protected].