April 1, 2007 (Vol. 27, No. 7)
Increased-throughput Spectrophotometry and Reduced Mass Required for Fluorescent Assay
Conservation of rare and low-yielding biological samples has become increasingly important as investigators routinely work with methods using progressively smaller amounts of isolated sample. Novel spectroscopic technology has enabled absorbance and fluorescence measurements using sample volumes of just 1 µL. The current state of microsample analysis has progressed in the areas of higher throughput spectrophotometry and limited-mass fluorometry. The ability to concurrently measure multiple 1-mL samples is addressing the demand for increased throughput using absorbance spectroscopy. Also, the development of small-volume fluorescence assays for use with microsample detection techniques is dramatically reducing the amount of material needed for fluorescence analysis. By combining microvolumes with the sensitivity of fluorescence, detection of as little as 2 pg of dsDNA is possible.
Increased-throughput absorbance analysis of minute samples and reduction in total mass required per fluorescent assay represent the latest breakthroughs in microvolume quantitation technology.

Figure 1a
A New Stage in Microvolume Analysis
A novel sample retention system developed by NanoDrop Technologies (www.nanodrop.com) allows absorbance and fluorescence analysis of 1-µL samples without the use of cuvettes or capillaries. This technology greatly reduces the amount of time required for spectral analysis by removing time-consuming steps such as cleaning cuvettes and preparing dilutions. However, performing spectral analysis one sample at a time is often impractical when many samples are involved.
Investigators involved with high sample turnover, such as genotyping facilities and molecular repositories, not only require conservation of sample for downstream application, but often handle hundreds or thousands of samples at any given time. Although the advent of 1-µL spectrophotometry allows microvolume quantitation with maximum conservation of sample, the limited throughput is often an issue for these types of laboratories. Consequently, some investigators simply forgo desired quality-control checks or apply assumptions based on sampling.
Many researchers use fluorescent plate readers that provide higher-throughput capability, but they are often displeased with the inherent variability of the results, the sample prep time required, and the amounts of material consumed in each reaction.
To address this issue and further improve lab productivity, NanoDrop Technologies recently developed the NanoDrop® ND-8000 8-Sample Spectrophotometer that allows for higher throughput than the NanoDrop single sample spectrophotometer. Using an eight-channel pipettor, 1-µL samples are pipetted directly onto the sample stage that consists of an array of eight optical surfaces (Figure 1a).
The eight-sample UV/VIS spectrophotometer then uses the inherent surface tension to capture and hold the 1-uL samples between the upper and lower optical surfaces during the spectral readings (Figure 1b).
The entire measurement cycle for 8 samples (sample loading, spectral reading, and sample removal) takes less than 30 seconds. Using this new system, researchers can now perform quality-control checks at critical points throughout workflows in ways that are either difficult or infeasible using single sample spectrophotometers or plate readers. Incorporating more quality-control measurements leads to a higher degree of confidence in the validity of data and ultimately improves productivity.

Figure 1b
Versatile Microsample Fluorometry for Ultralow Mass Detection
Advances in microvolume fluorometry are improving options for quantitation and downstream applications. The NanoDrop® ND-3300 Fluorospectrometer uses the same cuvetteless sample retention technology described above to hold a single 1–2 µL sample and measure the fluorescence emission spectrum in less than ten seconds (Figure 2). Fluorophore excitation occurs from one of three light-emitting diodes (LEDs): UV, blue, or white. The use of the broad spectrum, nonfiltered white LED is enabled by the combination of direct coupling of the sample to the optics and signal-processing technology. The broad excitation range allows for a wide range of common fluorophores to be measured (without the need for filter changes or a monochromator). This system provides versatility for experienced fluorescence users and simplicity for investigators less acquainted with fluorescence-based techniques.
Microvolume fluorescent measurement capability allows widely used nucleic acid quantitation assays, such as PicoGreen® (dsDNA) and RiboGreen® (RNA) assays, to be scaled down to small total reaction volumes such as 10 µL. This reduced reaction volume provides a substantial reduction in total sample mass required when compared to that required for plate readers and traditional 1-cm cuvette fluorometers. While microvolume fluorometry may not measure ultra-low concentrations, it does lower the fluorescent detection limit of sample mass by more than an order of magnitude.
For example, using the PicoGreen dye, the microvolume fluorometer can detect as little as 2 pg dsDNA, while a cuvette or microplate PicoGreen assay needs a minimum of 25–50 pg dsDNA for detection. When working with samples of limited biomass (such as with microgenomics and microproteomics), lowering the total mass needed per measurement is more important than the ability to measure samples of low concentration. Figure 3 illustrates the different detection limits of the microvolume fluorometer as compared to plate readers and cuvette-based systems.
Initial field studies have shown that the ability to quantitate total RNA extracted from individual Laser Capture Microdissection (LCM) samples using the 10-µL reaction volume has the potential to eliminate the practice of sample pooling thus allowing analysis of individual samples. Pooling samples is often necessary in order to obtain the minimum sample mass required for traditional larger volume plate readers or cuvette-based fluorometers. By reducing the amount of sample mass required for analysis, researchers save time acquiring initial material of interest, which often involves using costly time-consuming isolation and extraction techniques. By greatly reducing the total reaction volume of a fluorescence assay, less sample is required for basic quantitation, conserving the majority of the sample for downstream applications.
In addition to basic quantitation assays, such as PicoGreen and RiboGreen, the microvolume fluorometer is capable of performing more sophisticated fluorescent measurements. Excitation across a broad wavelength range enables the emission profile of several fluorophores in a single measurement. The broad spectral output lends itself to FRET analysis for homogeneous fluorescent assays. FRET assays are widely used throughout the scientific community for confirming the existence of a specific target, as well as revealing the interaction between molecules.
Microvolume FRET analysis was conducted with a FITC/Cy5 oligo nucleotide using the filtered 470 nm LED in the presence and in the absence of complementary target sequence. FRET oligo alone produced FITC (donor) fluorescence with no significant signal at the Cy5 (acceptor) emission wavelength (680 nm). Once complimentary sequence target was introduced and hybridized to the probe, a marked increase of Cy5 signal and decrease in FITC fluorescence was observed.
Molecular Beacon probes are similar to FRET oligo probes yet differ by using a nonfluorescent quencher to replace the acceptor. In the absence of a target sequence, the molecular beacon resides as a “stem loop” conformation via an intra-complimentary sequence allowing the quencher to come in close proximity to the fluorophore and suppress the fluorescent signal. The inherent single fluorophore/quencher construction of the molecular beacon allows the user to take full advantage of all three excitation sources, the most versatile of which is the broad range white LED (465 nm–650 nm).
Moreover, utilizing the white LED source for excitation enables the measurement of multiple molecular beacons within the same sample.
Researchers at the Public Health Research Institute in Newark, NJ, have successfully measured molecular beacons utilizing the microvolume fluorescence technology. The quality of several HPLC purified molecular beacon probes were determined by comparing signal to background values. The probes were diluted to a concentration of 0.1 µM and measured in the absence (background) and in the presence (signal) of the complimentary target sequence. Comparable results were obtained on the ND-3300 using 75-fold less sample than the larger volume reference instrument.
The overall reduction of sample required for fluorescent experimentation using a microvolume fluorometer coupled with a broad excitation range allows for more information to be generated from a single measurement while conserving a majority of the stock sample for downstream applications.
Figure 2
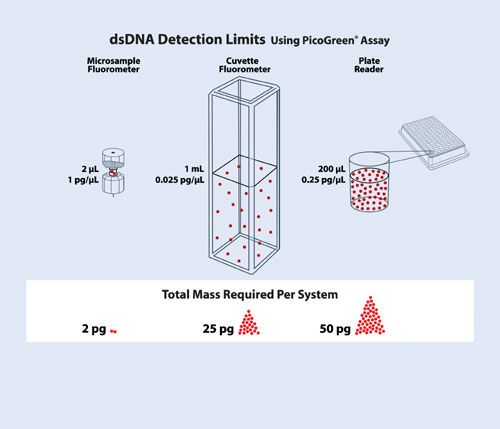
Figure 3
Molecular Beacon probes and their use require a license under patents owned by the Public Health Research Institute.
David Ash is application scientist and Philippe Desjardins is scientific marketing manager at NanoDrop Technologies. Web: www.nanodrop.com.
Phone: (302) 479-7707.
E-mail:[email protected].



