August 1, 2006 (Vol. 26, No. 14)
Improved Reporting for Agonists and Antagonists of GPCRs and Ion Channels
The bivalent ion Ca2+ is a critical second messenger involved in many physiological and signal transduction processes within a cell. The central role of Ca2+ in intracellular signalling, and its physical/chemical properties also makes it an attractive reporting molecule for drug discovery.
GPCRs, ion channels, and ion exchangers are among the most interesting target classes for the pharmaceutical industry. The majority of these target classes frequently trigger an intracellular Ca2+ mobilization upon activation with specific stimulating molecules. These reporting activities are commonly used in the search for agonists or antagonists of the target classes mentioned above.
The development of more sensitive and reliable technologies that measure transient changes in intracellular Ca2+ concentration continues to be an important research area. However, there are inherent challenges in developing precise Ca2+ mobilization assays for high-throughput screening (HTS) applications, since the Ca2+ release signal is rapid, short, transient, and sometimes weak.
The introduction of the Fluorometric Imaging Plate Reader (FLIPR, Molecular Devices), an image-based instrument capable of measuring 384 wells simultaneously, has allowed extensive use of fluorescent dye-based Ca2+ mobilization assays in the HTS process.
A valid alternative to the fluorescent-based Ca2+ mobilization assays is represented by the use of Ca2+-activated photoproteins with their inherent property of flash luminescence. The binding of Ca2+ ions to the active complex, consisting of the apo-photoprotein and coelenterazine, induces a conformational change, resulting in the oxidation of coelenterazine to coelenteramide with a subsequent emission of a blue light flash.
The use of photoproteins as indicators of Ca2+ activation in HTS offers many advantages: low background levels result in a large signal to noise ratio; Ca2+ concentrations can be measured at specific cellular sites; tested compounds only require short incubation periods; and reaction kinetics can be followed.
Aequorin, the first photoprotein to be isolated, has been validated for functional assays of many GPCRs, and the results obtained are comparable to those obtained with the use of fluorescent dyes. However, the adaptation of aequorin assays to HTS is not always easy due to the low quantum yield of aequorin and the fast kinetics of the reaction. Photoproteins capable of emitting a brighter signal and addressing targets other than GPCRs are therefore of importance for the HTS industry and could overcome some of the drawbacks of aequorin.
Axxam (www.axxam.com) has developed a Ca2+-activated photoprotein called Photina as a result of the development of reporter cell-based assays for HTS. Photina has the mechanism of action of a typical photoprotein, but the total light release is higher, and the reaction kinetic is slower.
Photina is a chimeric photoprotein created by replacing a portion of the obelin-coding sequence, located between EF-hand I and EF-hand II, with the corresponding part of the clytin sequence. To increase the expression for the chimeric protein in mammalian cells, a Kozak sequence was introduced, and codons were optimized for mamalian expression in regions with a very high (>80%) or with a very low (<30%) GC content.
The expression and functionality of Photina was tested in different cell lines frequently used in HTS processes, such as CHO and HEK293 cells. The results obtained showed that the expression of the protein is stable over time, does not affect cell growth and viability, and no toxicity is detected even after long periods of cell culture.
One of the most important differences between Ca2+-activated photoproteins and Ca2+ fluorescent dyes is that photoproteins can be transported to subcellular compartments of interest using the appropriate targeting sequences. As a result, Photina has been targeted to different cell compartments where the variation in Ca2+ concentrations has a particular physiological meaning, such as plasma membranes or mitochondria.
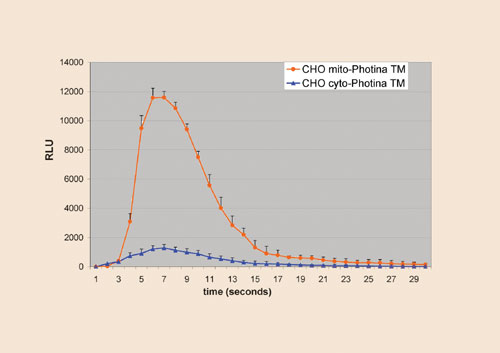
The localization of a Ca2+-activated photoprotein within the mitochondria has the advantage of amplifying the luminescent response triggered by the activation of GPCRs coupled to the Gaq/PLC pathway. Axxam has found that the transient flash luminescence is at least 10-fold higher than that obtained by the same stimulus applied on cells containing a cytoplasmic version of Photina (Figure 1).
Apart from monitoring GPCRs activity, Photina allows the signal detection for any cellular target that can increase the intracellular Ca2+ concentration. To assess this, several different targets like Ca2+ permeable ion channels or Na+/Ca2+ exchangers have been transfected in CHO cells that express either the mitochondrial or the cytoplasmic version of Photina. The results obtained clearly demonstrate that the functional activation of these targets can be detected easily and reliably by Photina.
The choice between cytoplasmic or mitochondrial localization of the photoprotein greatly expands the range of targets addressable by the use of flash luminescence. Generally, the cytoplasmic localization of Photina is more suited to the study of Ca2+ permeable ion channels and Na+/ Ca2+ exchangers, but there are cases in which the use of a mitochondrial localization is preferable (Figure 2).
For ion channels like the ionotropic purinegic receptors P2X1 or P2X3, instruments will generally miss the very fast activation kinetic, since the widespread cytoplasmic localization of Photina results in immediate detection of the Ca2+ wave of functional channel modulation. The same target could be more easily studied by transfecting it in a CHO cell line expressing the mitochondrial version of Photina. The spatially restricted localization of the photoprotein within the mitochondria allows a more accurate detection of the channel activation kinetic. This is due to the longer time needed for the Ca2+ wave to reach the mitochondria-localized Photina.

Fig.1: Luminescent assay on CHO cells stably expressing mito-Photina (red circles) and cyto_Photina (blue triangles)
Photina and HTS Plate Readers
The different cell-based assays expressing Photina and receptors of interest, such as GPCRs, ion channels, and Na+/Ca2+ exchangers, have been validated using most leading types of flash luminescence plate readers and with different plate formats. The results obtained show robust and reproducible light signals that present new insights and applications for photoprotein-based cellular assays for HTS. In all tested conditions, the light intensity generated by the Ca2+-activated Photina is high and allows the use of few cells/well compared with the usual quantity required by most other HTS protocols.
Figure 3 demonstrates that the signal intensity obtained with 250 cells/well is already strong enough on some instruments to guarantee reliable signal detection, implying that Photina has a light emission much stronger than most other known photoproteins. Photina is, therefore, particularly attractive for those HTS campaigns involving large compound collections, as the required production quantity of the cellular assay material is significantly reduced. An overall average Z’ factor value of 0.7 for most of the tested assays also provides the required robustness for application to HTS.

Fig 2a: Photina, and the very fast activation kinetics is not correctly detected by the instrument

Fig.2b: Photina in a longer time. This allows the correct recording of the activation kinetic.

Fig.3: Luminescence was monitored for 30 seconds after injection of ATP 500 nM.