February 1, 2016 (Vol. 36, No. 3)
Tissue Regeneration Is Moving Closer to Clinical Realization
The regeneration of tissues and body parts has fascinated people since the earliest times. The ancient Greeks, for example, told tales of Prometheus, the god who had his liver ravaged each day and restored each night, and of the Hydra, the monster that was capable of growing two heads for each head that it lost.
More recently, people have been inspired by advances in molecular biology and stem cell technology. These disciplines are introducing new ways of replacing diseased cells and generating new tissues. Today, the age-old dream of restoring broken bodies, making them whole, is tantalizingly close to medical reality.
“The translation of basic and regulatory science into the clinic is occurring now,” states Steven D. Schwartz, M.D., professor of ophthalmology and chief of the retina division at the University of California, Los Angeles. “I am optimistic that the scientific community will discover ways to diminish human suffering using stem cell biology and other regenerative strategies.”
Dr. Schwartz bases his optimism on his own experience conducting clinical trials that assess the ability of stem cell therapies to restore vision. He is just one of the experts represented in this article. He is accompanied by scientists who are, for example, advancing genomic correction strategies, mixed-cell-type approaches, integration optimization, and manufacturing best practices.
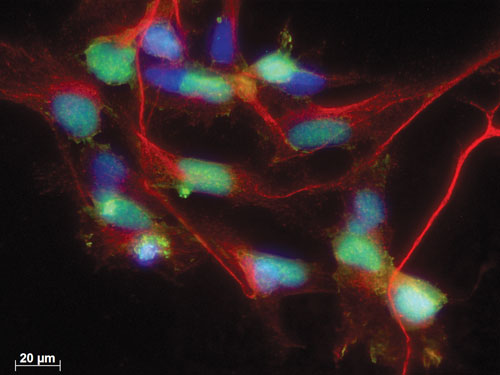
A research team spanning the New York Stem Cell Foundation and the Ying Liu Laboratory at the University of Texas Health Science Center at Houston optimized the targeting efficiency of the CRISPR/Cas9 system in a stem cell application. Specifically, they generated a dual knockin reporter in human induced pluripotent cells. Here, mCherry (red) expression faithfully reflects endogenous neurogenin 2 (green) expression during neural induction. Nuclei are revealed by DAPI staining. Scale bar: 20 µm. CRISPR/Cas9 reporter cell lines can be used to determine the role of transcription factors in human development and to purify neural lineage specific populations. [Ying Liu Laboratory]
Genomically Corrected Cells
“We have the tools to perform genomic correction and then differentiate cells into muscle precursor cells that can be used for transplantation,” says Guangbin Xia, M.D., Ph.D., assistant professor of neurology at the University of Florida College of Medicine. “We hope to move toward clinical trials.”
A major research effort in Dr. Xia’s group focuses on nucleotide repeat expansion neurodegenerative disorders. “For these conditions,” notes Dr. Xia, “induced pluripotent stem (iPS) cells hold great promise, not only for the stem cell transplantation therapies, but also for developing cellular models of drug screening and for conducting mechanistic studies.”
As part of its efforts to develop novel stem cell therapies, Dr. Xia’s group is focusing on spinocerebellar ataxias and myotonic dystrophy type 1 and type 2. For example, in one of its investigations, the group established iPS cells from patients with myotonic dystrophy type 1.
Myotonic dystrophy type 1, an autosomal dominant neurodegenerative condition, is caused by unstable CTG trinucleotide repeats in the 3′-untranslated region of the DMPK gene. Affected individuals harbor over 50 such repeats, and transcription of the mutated gene generates toxic RNA molecules that form nuclear foci and cause aberrant splicing, which explains the multi-system involvement that characterizes this condition.
“Correcting the mutated gene provides ideal cells for transplantation purposes,” asserts Dr. Xia.
By performing in vitro genome editing in neural stem cells derived from human iPS cells obtained from patients with myotonic dystrophy type 1, Dr. Xia and colleagues showed that the pathological accumulation of nuclear RNA foci can be reversed. “Our strategy involved incorporating polyA signals upstream of the repeat expansion to prevent transcription of the mutant transcript,” explains Dr. Xia.
These results provide the proof of principle for extending this strategy to generate other types of progenitor cells that can be used for autologous transplantation. “This approach,” notes Dr. Xia, “can also be applied to other nucleotide expansion neurodegenerative disorders.”
Cell replacement is a promising therapeutic intervention for patients who have advanced-stage neurodegenerative disorders and who have already lost large numbers of cells. A shared feature of many neurodegenerative conditions is the asymptomatic course that can sometimes span decades before the disease becomes clinically manifest.
In some conditions, such as Huntington’s disease, symptomatic onset may only occur in the fourth or fifth decades of life. Correcting the mutation during the asymptomatic stage promises to prevent the onset of clinical disease. “Similar to the in vitro approach,” concludes Dr. Xia, “we are working on developing an in vivo genome editing strategy that can be used at the preclinical stages and fundamentally cure the disease.”
Mixed Cell Types
“Our idea was that combining mesenchymal and cardiac adult stem cells would lead to better outcomes in stem cell therapy,” says Joshua M. Hare, M.D., professor of medicine and senior associate dean for experimental and cellular therapeutics at the University of Miami Miller School of Medicine. The potential therapeutic advantage of combining these two cell types originated from an observation that Dr. Hare and colleagues made years ago in an animal model.
In this short-term cardiac injury model, after injecting mesenchymal stem cells into a cardiac injury area, one of the dominant responses was a proliferation of endogenous cardiac stem cells. “We predicted that if these cells work interactively, we could enhance the therapeutic response by mixing the two cell types,” explains Dr. Hare.
Subsequently, Dr. Hare and colleagues conducted a follow-up study in animals with long-term injury, including myocardial scarring. “We obtained a synergistic response in two ways,” informs Dr. Hare.
While mesenchymal stem cells and the cell mixture had similar effects on reducing tissue fibrosis, the mixture improved cardiac function to a greater extent. Additionally, when the cell mixture was used for transplantation, more of the endogenous cardiac precursor cells from the injured area were found to be in the cell cycle.
“Our studies have shown safety and efficacy in the animals,” asserts Dr. Hare. “And we received approval for human clinical trials that will start within the next year.”
One of the challenges in stem cell therapies is that even if a desired outcome is accomplished from a physiological standpoint, this does not necessarily mean that the approach will become a successful clinical intervention. “The most significant gap that we are having in the field is a disagreement amongst scientists as to what the outcome should be,” states Dr. Hare. For example, some investigators believe that remuscularization, indicating that stem cells had differentiated into myocytes, should be achieved before proceeding with clinical testing.
“Others, including myself, have a different mindset,” insists Dr. Hare. “The main outcome that we consider is whether the strategy makes people better irrespective of how this occurs.”
The challenges that this gap opens for translational research have far-reaching therapeutic implications. “Unless we bridge that gap,” concludes Dr. Hare, “we will see a delay in the development of new therapies and clinical trials that are required to test whether they work.”
“The technology we adapted to trace circuitry integration of cells after transplantation uses a modified rabies virus that traces neuronal connectivity,” says Malin Parmar, Ph.D., professor of developmental and regenerative neurobiology at the Wallenberg Neuroscience Center and the Lund Stem Cell Center.
A key consideration in the stem cell research arena is to ensure that transplanted cells survive, mature, and can functionally replace diseased cells that have been lost. In neurodegenerative disorders, functionality of the transplanted cells is assessed by their ability to integrate into neuronal circuits.
“Techniques that examine the ability of transplanted neurons to establish synaptic connections have been available for some time,” notes Dr. Parmar. “But they are time-consuming and complex, and we wanted to establish a quick and easy approach.”
To develop a better tool for examining synaptic connectivity, Dr. Parmar and colleagues took advantage of the fact that the rabies virus normally infects neurons and spreads by retrograde transport across active synapses. In an engineered rabies virus, the gene encoding the viral glycoprotein, essential for the transsynaptic transmission of the virus, was replaced with the gene encoding the fluorescent marker mCherry. Also, a vector was used to carry the genes encoding nuclear GFP, the cellular receptor required for rabies virus infection, and the rabies virus glycoprotein. Ultimately, this vector served to mark the reprogrammed neurons.
In this experimental system, the mutant virus can spread by retrograde axonal transport across one synapse. As a result, reprogrammed neurons present a green nucleus and a red cytoplasm, while neurons establishing synaptic connections appear red as a result of the cytoplasmic expression of mCherry. “If a neuron becomes red,” advises Dr. Parmar, “it means that it has established a synaptic contact with the transplanted cells.”
Using this system in a rat model of Parkinson’s disease, Dr. Parmar and colleagues revealed that dopaminergic neurons derived from human embryonic stem cells integrated into synaptic circuits. The dual fluorescent marker system allowed the source of the afferent neuronal contacts to be identified, and it revealed that afferent synaptic contacts can be established as soon as six weeks after transplantation.
“We can now address questions that for a long time we have not been able to answer,” asserts Dr. Parmar. Some of these unsolved questions revolve around the effects that the location and the type of transplanted cells have on the success of the integration. “This experimental setting allows us to examine how long it takes for a transplant to get integrated and study the factors that affect this process,” concludes Dr. Parmar. “This will help generate better cells and develop better therapies for patients.”
Clinically Compliant Protocols
“One of the major challenges in stem cell research is to develop manufacturing protocols that are compliant with the current good manufacturing practices,” says Mahendra Rao, M.D., Ph.D., vice president for regenerative medicine at the New York Stem Cell Foundation. Efforts to develop such clinically compliant protocols involve several steps, including the stem cell manufacturing process, staff training, the design and maintenance of laboratory facilities, and validation and quality control testing.
In a recent study describing their ongoing efforts to define guidelines for manufacturing induced pluripotent cell master cell banks using good manufacturing practices, Dr. Rao and colleagues emphasized the need for a robust, reliable, and reproducible manufacturing processes that would cover multiple facets of the process, including cell culture, documentation, and quality control. Central to good manufacturing practices is the ability to efficiently and reliably generate biological reagents, sensors, and reporters.
In the case of stem cell research, genetic modification has become an increasingly indispensable technology for generating reporter cell lines. Commonly used gene-editing technologies include zinc finger nuclease, transcription activator-like effector nuclease, and CRISPR/Cas9 systems. “The advantage of CRISPR/Cas9 over the other two approaches is that it is inexpensive to perform and appears to be even more broadly applicable,” notes Dr. Rao. These are two reasons why it is becoming increasingly popular.”
Another advantage of the CRISRP/Cas9 system is the possibility of performing gene editing in stem cells and iPS cells without the need for subcloning. “This provides all the advantages of the lentivirus-based systems,” adds Dr. Rao. “At the same time, it offers the possibility of controlling targeting at a specific site and avoiding off-target effects.”
As part of a team that included Ying Liu, Ph.D., a researcher at the University of Texas Health Science Center at Houston, Dr. Rao optimized the targeting efficiency of the CRISPR/Cas9 genome-editing system and generated a dual knockin human iPS cell reporter for neurogenin 2, a gene important for the development of the central nervous system. For this gene, using the CRISPR/Cas9 targeting system, Dr. Rao and colleagues accomplished a 33% targeting efficiency, which is about 10-fold higher than the one that can be obtained with homologous recombination-based targeting approaches.
“The patent landscape for CRISPR/Cas9 is not as clear-cut as for the zinc finger nuclease technology,” observes Dr. Rao. “But perhaps because of this, many more people are willing to use it.”
Clinical Trials
Among the major causes of vision loss, blindness, and disability are age-related macular degeneration, a progressive eye condition that mostly affects individuals over 50 years old, and Stargardt’s macular dystrophy, the most common form of inherited juvenile macular degeneration. Stargardt’s affects children and young adults.
In two recent Phase I/II prospective clinical trials, Dr. Schwartz and colleagues delivered human embryonic stem cell–derived retinal pigment epithelium to the subretinal space in patients with atrophic age-related macular degeneration and Stargardt’s macular dystrophy. The transplanted cells were well tolerated by the participants for up to 37 months without serious safety concerns. In both patient groups, pigmented tissue was visualized after transplantation at the border of the atrophic lesions, and their density and size increased with time after surgery.
These findings provided the first evidence on medium- to long-term safety after pluripotent stem cell progeny transplantation in any human disease. “We have to conduct more human trials to carefully study in a controlled manner dosing, delivery, target populations, and immunosuppression,” says Dr. Schwartz. “If performed in a timely fashion, these interventions may reduce or reverse blindness, improve quality of life, and prolong life.”



