August 1, 2015 (Vol. 35, No. 14)
Application of the SFR vario in Prokaryotic and Eukaryotic Systems
A novel system for online biomass measurements inside shake flasks has been developed based on near 360° scattered light detection. The optosensoric components for biomass monitoring were combined with established PreSens Shake Flask Reader (SFR) technology for read-out of optical sensors inside the shake flasks.
This new analytical unit can now be employed to monitor biomass, pO2, and pH simultaneously and contactless through the flask bottom. First application of the prototype SFR vario (Figure 1) on Escherichia coli K12 and Kluyveromyces marxianus culture resulted in highly reproducible online biomass data, with relative errors matching those of standard offline optical density (OD) measurements.
In early stages of bioprocess development, shake flasks are often applied because results can be achieved with as little effort as possible by running many experiments in parallel. At this small scale, however, it is difficult to monitor or control important cultivation parameters. Cell growth determination in shake flasks, for example, requires offline sampling. This is a major drawback as it raises the risk of contamination and increases the workload.
Furthermore, these measurements can be performed only at distinct points in time, leaving huge gaps, missing information that could be decisive for deeper process understanding. Now a new analytical unit—the SFR vario—has been developed for online monitoring of cell growth in shake flask culture. The biomass measurement is based on detection of light scattered by particles inside the liquid.
Through the use of nonlinear calibration models, a correlation between (optical density (OD) and cell dry weight (CDW) can be established. The optoelectronic components used for cell growth determination are implemented in the established SFR system to create a monitoring platform that allows simultaneous pO2, pH, and biomass measurements in the shake flask cultures. Initial evaluation tests with E. coli K12 as prokaryotic and K. marxianus as eukaryotic models were performed providing highly reproducible results.
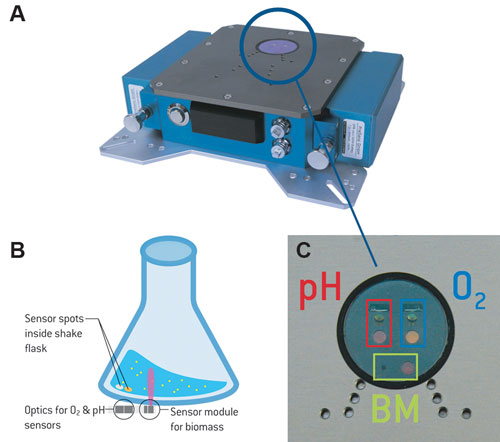
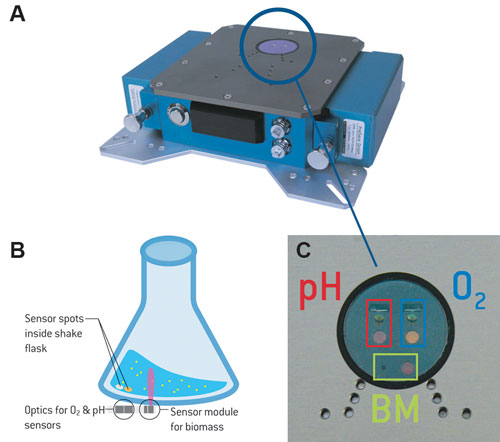
Figure 1. SFR vario prototype (A); schematic illustration of the measurement principle (B); SFR vario optics for biomass (BM), O2, and pH measurements (C).
Materials & Methods
An additional sensor module for determination of scattered light with a scatter angle of about 360° was incorporated in the optics for pO2 and pH measurement of the SFR. The ray of light emitted by an LED is transmitted through the flask bottom and scattered by particles (cells) inside the culture medium; this scattered light is detected by a photodiode. During shaking, a liquid sickle forms inside the shake flask, so a piezoelectric acceleration sensor was integrated to optimize the measuring cycle. It can adjust the time between measurements.
The performance of this new analytical system was demonstrated with E. coli K12 and K. marxianus. E. coli was cultivated in minimal medium containing glucose and lactose at 37°C to follow diauxic growth. K. marxianus was cultivated in YM-medium with glucose monohydrate at 30°C. Both microorganisms were cultured in 500 mL baffled shake flasks with integrated pO2 and pH sensors (SFS shake flasks) at a 100 mL working volume and 150 rpm.
All cultivations were carried out four times under the same conditions. Three were used to generate a calibration model of scattered light intensity as a function of OD600. Therefore, offline samples were taken every 60 min under sterile conditions, and OD600 was determined. The remaining cultivation was used for validation. Measurements of biomass, pH, and pO2 were taken with a sampling interval of 15 seconds.
Online Biomass Monitoring with the SFR vario
Before the SFR vario can be used for online biomass monitoring, a valid correlation between scattered light and biomass concentration explicitly for the monitored cell type in the respective cultivation conditions had to be deduced. Therefore, offline OD600 measurements of the samples were correlated with the respective online measured values. For E. coli K12 and K. marxianus, this correlation can be best described by a simplified Bleasdale–Nelder function:
y = (a + b · x)(−1/c)
where y is the OD600 value; x stands for the measured light intensity; and a, b, and c are the respective median filtered parameter values determined for the specific cell type.
Figures 2 & 3 show biomass measurements with the prototype SFR vario in E. coli K12 and K. marxianus culture. Additionally, the online measurements of oxygen and pH as well as the offline determinations of OD600 and substrate concentration are depicted. The sampling rate for biomass measurements was set at 15 seconds, so a large amount of data was obtained, giving a good real-time overview of the biomass development.
At the beginning of the cultivation, some measurement noise was observed in cultures of both microorganisms. This is caused by low starting cell densities and boundary layer reflection of the detection light beam. With the cell density growing and the culture broth becoming sufficiently turbid, the biomass measurements stabilized and started increasing. A median filter was applied to smooth the graphs. The interruptions in oxygen and pH measurements were caused by stopping the shaking movement to take samples for offline measurements.
In Figure 2, the diauxic growth of E. coli can be clearly determined form the measurement values provided by the prototype device. After glucose in the medium is consumed, a metabolic shift from glucose to lactose consumption takes place. It can be observed in a small plateau in the biomass measurements. Moreover, the exact match of stopped growth with rapidly increasing oxygen levels as well as a plateau in pH measurements could be recorded.
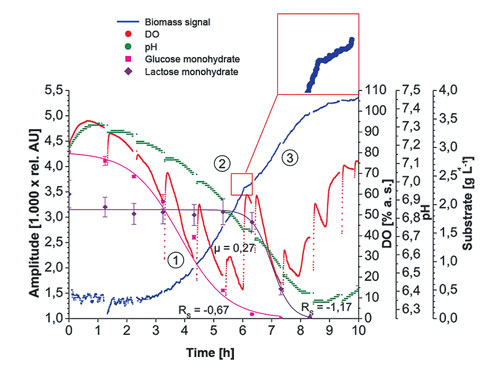
Figure 2. Diauxic growth of E. coli K12 in minimal medium with glucose and lactose: online biomass (median 45), dissolved oxygen (DO), and pH measurements recorded with the SFR vario prototype. Additionally, offline measurements of OD600 and substrate concentration are depicted. Insert: Ceased growth during the metabolic shift from glucose to lactose consumption, visible in a plateau in the biomass measurements.
The different growth phases of K. marxianus can also be analyzed with online biomass data. In the first phase, glucose is metabolized under aerobic conditions. When glucose in the medium becomes limiting, the cell metabolism switches to metabolize the products of the previous glucose consumption under high oxygen demand. This phase shows a lower growth rate than in the previous phase. The difference is clearly visible in the biomass measurements.
Due to oxygen limitation, growth is linear in this phase. So with the prototype SFR vario, we were able to follow biomass development inside shake flasks of eukaryotic and prokaryotic microorganisms and gain measurement results of a kind never recoded before by like means in shake flasks. With the data provided by the SFR vario, all relevant information about culture conditions can be monitored without the need for offline analysis.
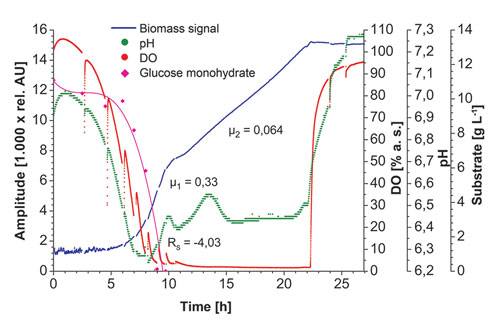
Figure 3. Growth phases of K. marxianus culture in YM-medium inside shake flasks: online biomass (median 45), dissolved oxygen (DO), and pH measurements recorded with the SFR vario prototype. Additionally, offline measurements of OD600 and substrate concentration are depicted. Slower growth during the second phase is clearly visible in the biomass measurements.
Conclusion
We successfully demonstrated the functionality and potential of this new sensor system by online monitoring of different cell types. Biomass measurements with the SFR vario prototype can be conducted in both prokaryotic and eukaryotic cell suspensions. High accuracy can be obtained with an error of less than 12% compared to alternative offline techniques, as shown by the calibration models for E. coli K12 and K. marxianus.
Investigations with further cell lines and culture conditions are already underway, and it is becoming evident that online measurements of pO2, pH, and biomass with the SFR vario can be applied in a versatile application range.
Individual calibration models deduced for different cell types and culture conditions are delivering precise predictions of the biomass concentration. By using this sensor system for contactless measurements through the flask bottom, it is possible to get information about cell growth in shaken cultures in real time.
Jörg Schmidt-Hager, Ph.D., Christian Ude, Thomas Sheper, Ph.D., and Sascha Beutel, Ph.D., are affiliated with the Institute of Technical Chemistry, Leibniz University of Hanover, Germany. Michael Findeis and Gernot T. John, Ph.D. ([email protected]), are affiliated with PreSens Precision Sensing, Regensburg, Germany.