February 15, 2016 (Vol. 36, No. 4)
Michael Artinger, Ph.D. Vice President of Marketing ViroCyt
Michael W. Olszowy, Ph.D. Chief Technology Officer ViroCyt
Rebecca Montange Ph.D. Staff Scientist ViroCyt
ViroTag Allows Biologically Relevant In-Process Monitoring of Total Particle Count
Following notable setbacks during the previous decade, the gene therapy field is resurgent, both in the breadth and the depth of different applications being pursued using this approach. With its success, however, comes the realization of the challenges inherent with transitioning from early research and development to late-stage clinical trials and product launch. A primary issue is the consistent and compliant production of viral vectors at levels necessary to support commercialization. As the number of successful trials grows and the likelihood of multiple product approvals increases, the realization that current manufacturing technologies have not kept pace highlights this as an area in urgent need of innovation.
One of the most problematic steps is in the quantification of viral vectors during growth, harvest, purification, and release. Current methods like quantitative PCR and absorbance readings at 260 nm and 280 nm are highly variable, resulting in over- or under-estimation of particles present at any given step. The ramifications for manufacturing are lost product, delays, and cost overruns, which are serious, yet pale in comparison to the risks associated with administering too little (no therapeutic effect) or too much (adverse immune response) product to patients. Clearly, there is a critical requirement for a rapid, more precise means of quantifying viral particles used for vector-mediated gene therapy.
ViroCyt® is a Colorado-based biotechnology company focused on enabling real-time enumeration of viruses across multiple application areas. This includes viral vaccines, virus-based expression systems, and viral vector production. The primary method of detection utilizes a fluorescently labelled antibody specific to the virus of interest. ViroCyt has developed a number of these reagents under the trade name ViroTag®, and continues to launch new versions addressing the most relevant and important viruses.
In this assay, 5 µL of the ViroTag solution are added to 195 µL of the virus preparation and incubated for 30 minutes at room temperature, followed by a one-minute analysis using the Virus Counter® 3100.
In the instrument, the number of unique fluorescent events above an automatically determined threshold yields the amount of virus particles per milliliter volume (vp/mL). Due the high degree of specificity of ViroTag for the virus, both crude, partially purified, and fully purified samples are amenable to this approach, allowing evaluation throughout upstream and downstream processing.
To demonstrate proof of concept for the quantification of viral vector particles, two of the most prevalent viruses currently in development were selected for evaluation: adenovirus and adeno-associated virus.
Adenovirus
Adenovirus (AD) is nonenveloped, with a 90–100 nm icosahedral nucleocapsid containing a double-stranded DNA genome ranging from 26 to 48 kbp in size. With over 400 trials in progress, AD is one of primary vehicles for delivering genetic payloads to specific cell types, especially neoplastic cells. Because AD is known to cause respiratory disease in humans, primarily in children, it is essential that key viral genes are replaced with the required therapeutic gene or genes. To avoid this issue, as well as the likelihood of a host immune response to the vector, non-human strains of AD are also being investigated.
This point, especially as it relates to quantification, has been specifically called out by the FDA: “Given the potential toxicity of the adenoviral particles themselves, CBER recommends that patient dosing be based on particle number.”1
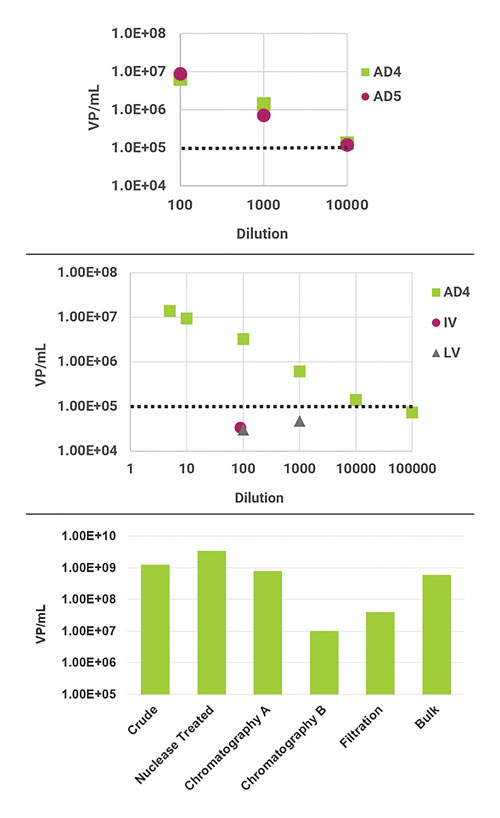
ViroTag ADVX (adenovirus, cross-reactive) uses an antibody capable of detecting multiple Adenovirus serotypes and has been tested with AD 2 through 6 on the Virus Counter 3100. Figure 1 demonstrates the results for dilution series of Adenovirus 4 and 5, as well as the specificity of ViroTag ADVX using unrelated viruses. Also included in this figure is an example of data obtained for in-process AD samples.
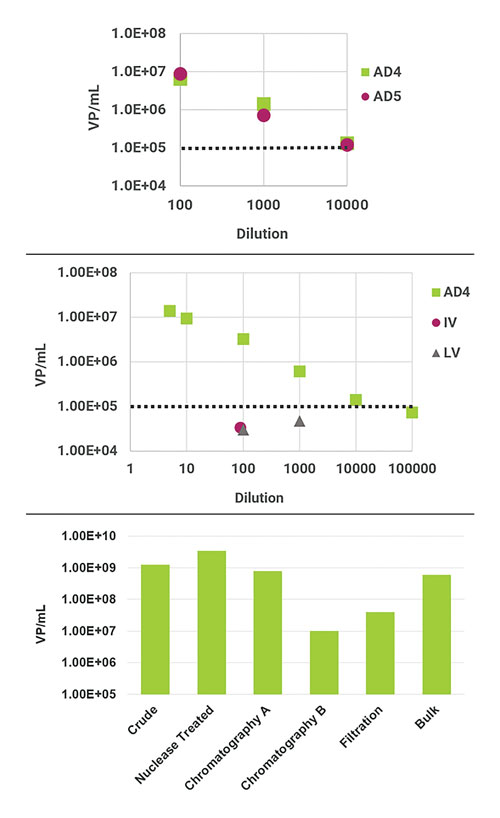
Figure 1. (A) Stock samples of adenovirus serotypes 4 and 5 were quantified using ViroTag ADVX on the Virus Counter 3100 and show good linearity with dilution. (B) The ViroTag ADVX reagent detects adenovirus (AD) and not influenza (IV) or lentivirus (LV) above the Virus Counter 3100 lower limit of detection. (C) Quantification of in-process adenovirus samples by ViroTag ADVX. Dotted lines represent the lower limit of detection of the Virus Counter.
Adeno-Associated Virus
Adeno-associated virus or AAV, is also a nonenveloped virus, albeit much smaller than AD, with a particle size of 25 nm and a 4.7 kb single stranded DNA genome. The principal advantages of AAV as a viral vector are the absence of pathogenicity, low host immune response, and long-term expression.
A noteworthy shortcoming, however, is the limited genetic capacity of the small particle. There are currently in excess of 100 clinical studies underway using AAV-mediated gene therapy across a broad spectrum of diseases, including hemophilia, heart disease, muscular dystrophy, cystic fibrosis, and Alzheimer’s disease.
While the most-often-used method for quantifying AAV is quantitative PCR, it is generally recognized in the AAV community that qPCR is at best an indirect readout of particle number, and results for the same sample can vary by as much as 3x when performed on the same instrument and even more (up to 10x) when the sample is analyzed on different hardware and/or by different laboratory personnel.
The ability to use qPCR is also often confounded by the aberrant packaging of constructs in excess of the normal 4.7 kb genome size. Because qPCR typically underreports the copy number—and by inference, particle count—concerns around injecting higher than necessary amounts of AAV particles are well-founded.
Thus far, ViroTag reagents have been developed for four different AAV serotypes (2, 3, 5, and 8), each of which was selected based on two primary criteria: importance as a viral vector and availability of well-characterized and highly specific monoclonal antibodies. All clones were chosen for their abilities to bind only intact capsids, irrespective of genome content. As is shown in Figure 2, ViroTag AAV2-3, which was developed to detect both AAV2 and AAV3, was evaluated for cross-reactivity and specificity both within the AAV family and across more divergent viruses.
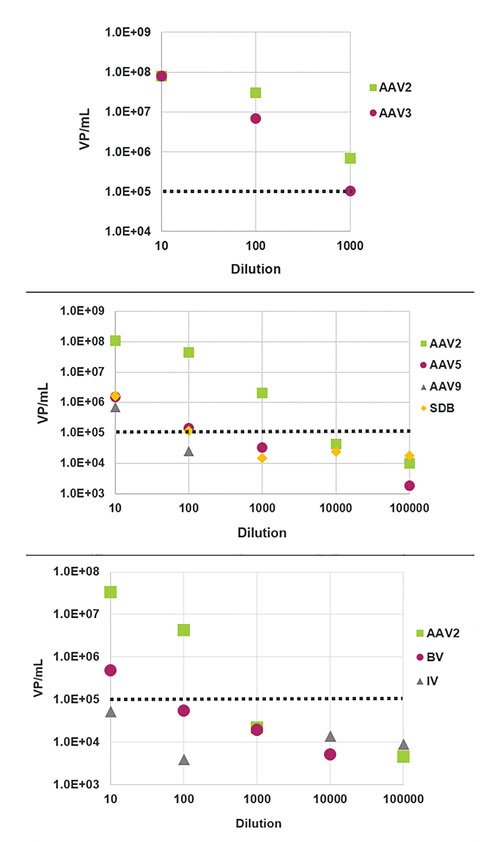
Figure 2. (A) Crude samples of AAV2 and AAV3 were quantified using ViroTag AAV2-3 on the Virus Counter 3100 and show good linearity with dilution. (B) The ViroTag AAV2-3 reagent does not cross-react with either AAV5, AAV9, or sample dilution buffer (SDB) above the Virus Counter 3100 lower limit of detection, except in the least-diluted sample, most likely due to mass effect. (C) ViroTag AAV2-3 does not detect baculovirus (BV) or influenza (IV) above the Virus Counter 3100 lower limit of detection, except in the least-diluted sample, again most likely due to mass effect. Dotted lines represent the lower limit of detection of the Virus Counter.
Conclusion
The lack of robust quantification methods for viral vectors is a significant unmet need in the gene therapy arena. Direct, real-time, and specific titration of viral particles during growth, purification, final quality control, and release is essential for consistent, efficient, and scalable manufacture of safe product. ViroTag, when combined with the Virus Counter 3100, provides an immediate readout of particle count regardless of the purity of the sample, permitting the benefits of in-process monitoring such as optimizing growth conditions, maximizing yield, and identifying problems as soon as possible.
References
1. Guidance for Human Somatic Cell Therapy & Gene Therapy, FDA Centers for Biologics Evaluation and Research, 1998.
Michael Artinger, Ph.D. (martinger@viro?cyt.com), is vice president of marketing and strategic partnering, Rebecca Montange, Ph.D., is staff scientist, and Michael Olszowy, Ph.D., is chief technology officer at ViroCyt.