May 15, 2012 (Vol. 32, No. 10)
System Developed for the Rational Design of Expression Plasmids
Efficient production of pharmaceutical proteins has become a necessity for both the biosimilars industry as well as for the development of new drug targets/proteins.
For the latter, the identification and screening of new targets requires a production level that warrants the economics of clinical development. Increasing the yield for targets otherwise discarded will result in a broad pipeline, thus boosting the chance of successful market introduction.
For biosimilars the costs of production are an important factor determining the profit margin of the marketed product. Ramping up production yields can save millions of dollars per year.
The production system of choice remains the CHO cell culturing platform, for which a wealth of innovations has been implemented to improve production of pharmaceutical proteins. Maximizing the yield of the production process has been a particular focus, and titers have increased over the last decade.
Still, further improvement is required and possible, both in the field of process development and downstream processing, as well as in the area of cell-line optimization.
Pichia Platform
The discovery and rapid exploitation of the Pichia platform demonstrates that this type of yeast is a promising alternative to the mammalian culture approach. Unfortunately, its protein glycosylation pattern differs from mammalian/human cells. Both bioindustrial and academic scientists are working hard to circumvent this drawback.
The advantages of Pichia are the ability to grow to high cell density and the presence of an efficient growth-to-protein production switch. For this organism, optimization of its protein production machinery is less developed.
The process of secreted recombinant protein production by both mammalian and Pichia hosts follows the same straightforward pathway—transcription, translation, and post-translational modification. ProteoNic contributes to yield improvement by focusing on the translation step.
The translation process can be divided into three sub-processes: initiation, elongation, and termination. The initiation phase is the rate-limiting step. It starts when a variety of proteins binds to the mRNA and to parts of the translation machinery (the ribosome subunits). Upon completing the proper binding sequence, translation begins, and the fully functional mRNA-ribosome complex will start to build the peptide chain.
Elongation of the peptide occurs by the translocation of the ribosome step-by-step along the mRNA. At each point new amino acids bind to the nascent protein. Termination follows as the code in the mRNA orders the ribosome to stop adding more amino acids. The protein is then released, and the protein synthesizing machinery is carefully disassembled and recycled.
The freshly produced proteins often need further processing to become fully functional and/or transported. These steps will not be considered in this review.
Few genetic technologies have been reported to increase the translation yield or the translatability of mRNA. The only commonly used technique is codon optimization in which the genetic code of a gene of interest is adapted to the presence of the tRNAs that are most used by the cell.
mRNA expression per se was found to be a poor predictor of protein levels as a low-copy, efficiently translated mRNA molecule can easily out-compete a high-copy, poorly translated mRNA. This is exemplified by the fact that the ratio of protein molecules to mRNA molecules in a cell depends on the protein concerned and this ratio varies by at least three orders of magnitude. The beginning (the 5´ end) of the mRNA is important for regulating translation because its structure affects ribosome recruitment and scanning.
Case Studies: CHO and Pichia
Most pharmaceutical proteins are produced using the mammalian cell as a production host. We set out to improve the translation process. In this example, commercially relevant proteins were used to evaluate the benefit of translation enhancement elements (TEEs).
We also used a cell system in which the composition of the gene of interest is the only variable and in which the host cells are genetically identical. In this expression system (the so called Flp-In system) the transgenic cells are made by targeted single-copy integration. Thus, either a gene was incorporated with the coding sequence in the standard genetic context (CMVpromoter-5´UTR-coding sequence-3´UTR) or in a context in which the UTR (but not the promoter or coding sequence) was altered.
Using a variety of pharmaceutically relevant proteins, e.g., interleukin 4, we found that the amount of protein molecules produced per mRNA is increased by a factor of 1.7, while the yield is increased by a factor of almost 3. Since the protein stability is not altered in these experiments, this implies that the yield increase is also a result of increased mRNA concentration.
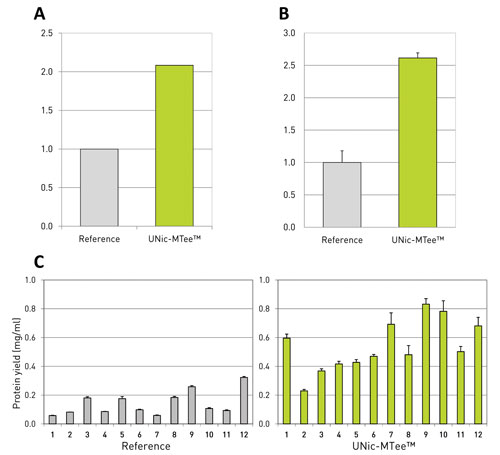
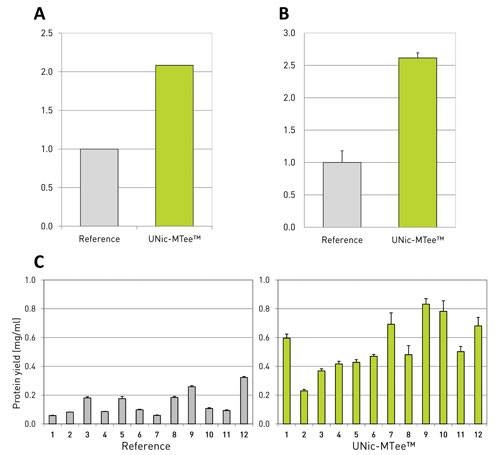
Figure 1. Beneficial effect of translation enhancement elements (TEEs) in CHO cell lines: Panel A shows translation efficiency, defined as protein per mRNA, whereas panel B shows the total protein yield. The references were set at one. Panel C presents the single subclone IgG expression levels. Comparison of the 12 best producing single clone cell lines with and without the UNic technology.
Most likely this concentration increase is caused by higher stability. This implies a better usage of generated mRNA by the altered regulating sequence (Figures 1A and 1B). ProteoNic’s UNic-MTee™ system was also successful in a client setting—picked stable clones that contained UNic-MTee showed an over twofold increase in IgG production (Figure 1C).
In Pichia we applied the commonly used Invitrogen pPIC9K-GS115 expression system (Life Technologies). The vector was constructed to express human interleukin 8 (hIL-8). Next, we replaced the AOX-UTR from this reference construct, which is located between the AOX promoter and the alpha mating factor signal sequence, by either UNic-YTee 1 or UNic-YTee 2.
After transformation 11 random clones of each construct were analyzed for yield. Both UNic-YTee 1 and UNic-YTee 2 result in more transformants that have an increased interleukin production. The yield increase is a factor of 5 to 7, both in 25 mL shake flask (Figure 2A) and 0.5 L bioreactor (Figure 2B) conditions.
The track record of CHO cells in the production of pharmaceutical proteins ensures its use in cell-line generation programs for many years to come. Significant improvements in cell-line generation and culture technology have resulted in acceptable production levels routinely.
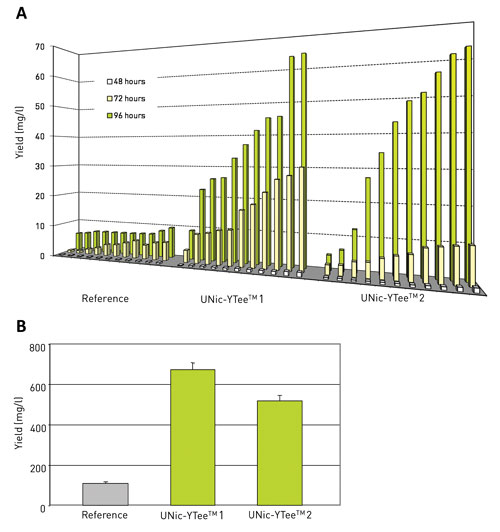
Figure 2. Contribution of UNic translation enhancement elements to the yield of human recombinant interleukin 8: Panel A: Eleven Pichia transformants harboring either the original AOX UTR-sequence (Reference), or a UNic-YTee translation enhancement element (UNic-YTee 1 and UNic-YTee 2, respectively) were analyzed for secreted hIL-8 yield. Panel B: The best transformant of each series was subjected to a bioreactor fermentation using Invitrogen’s recommended minimal methanol medium. The yield data of duplicate methanol fed runs were collected after 96 h induction. Other conditions: pH=6, 30°C.
Maurice van der Heijden, Ph.D., is research manager, Victor Schut ([email protected]) is CBO, and Raymond M.D. Verhaert, Ph.D., is CSO at ProteoNic.