February 15, 2006 (Vol. 26, No. 4)
The technology of RNA interference has rapidly evolved as a revolutionary tool for studying gene function, biological pathways, and the physiology of disease. Work and refinement of the RNAi technology has exploded in recent years. We now know the basic mechanism of the endogenous RNAi pathway, that the pathway is present in most eukaryotes, and how cellular machinery can be harnessed to silence gene expression.
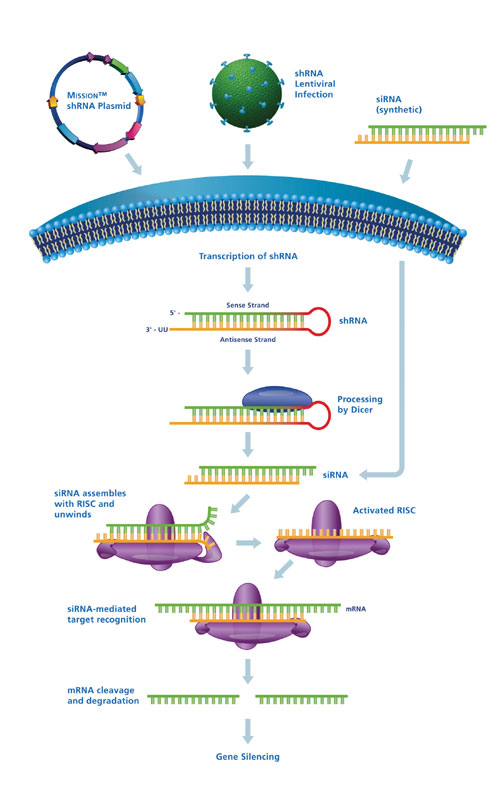
Key rules and parameters for use of siRNAs less than 30 base pairs (bp) were developed that allow use of dsRNAs in mammalian cells without triggering the cells antiviral response mechanism. Upon transfection, synthetic siRNAs by-pass cleavage by the RNase enzyme Dicer (Figure 1) and are taken up by the RNAi-Induced Silencing Complex (RISC). RISC unwinds the double-strand siRNA and the activated complex with the associated antisense siRNA strand targets the homologous mRNA transcript for cleavage and subsequent degradation. The reduction in transcript level results in lowered levels of the target protein, resulting in phenotypic changes.
Gene silencing or knockdown can be assayed at the mRNA transcript level using methods such as qRTPCR or at the protein level via western blotting, ELISA, and, more recently, mass spectrometry-based protein quantitation methods.
siRNAs can be expressed from DNA vectors within the host cell, providing methods for longer-term silencing, inducible silencing, and a plasmid DNA format that can be replicated for unlimited supply. These vector-based RNAi platforms may be integrated with viral delivery systems allowing gene knockdown in a myriad of cell lines.
Recent studies of endogenous miRNAs suggested that synthetic miRNA mimics could be used to induce the RNAi pathway rather than directly using the standard 21bp siRNA sequence. These synthetic forms of miRNA, termed short hairpin RNAs (shRNAs), are expressed from pol II or pol III promoters. The hairpin structure is recognized and cleaved by Dicer to form siRNA that are subsequently taken up by RISC for silencing of the target gene.
Methods for designing optimal shRNAs are similar to those for designing siRNAs. However, shRNAs are designed as inverted repeats that produce an intramolecular stem-loop structure upon expression. The stem structure is typically 1929 bp while the loop length also varies. Upon cleavage of the shRNA by Dicer, the stem provides the sense and antisense strands of the resulting in vivo processed siRNA. The use of synthetic siRNAs and vector-based shRNAs provide complementary approaches and solutions depending upon the experimental system being studied.
Discussion
As the portfolio of RNAi methods continues to expand, options become available for even the most complex systems being studied. Until recently, synthetic siRNA was the RNAi vehicle most broadly applicable to a wide variety of systems and applications. With commercial suppliers designing and producing synthetic siRNAs, little manipulation is required for the consumer. This format is amenable to any scale of research being performed provided the system is easily transfected (e.g., standard transformed cell lines).
Obstacles for using synthetic siRNAs include being a nonrenewable resource, the transient nature of silencing, and the difficulty faced in transfecting primary cells and nondividing cell lines such as neurons, lymphocytes, and macrophages. In addition, in vivo knockdown studies are particularly cumbersome. For those facing these hurdles, DNA vector-based shRNA methods provide the necessary solutions. shRNA expression vectors may be propagated in Escherichia coli and thus provide an unlimited supply of DNA for transfection. In addition, such vectors provide selectable markers for stable shRNA expression and gene silencing.
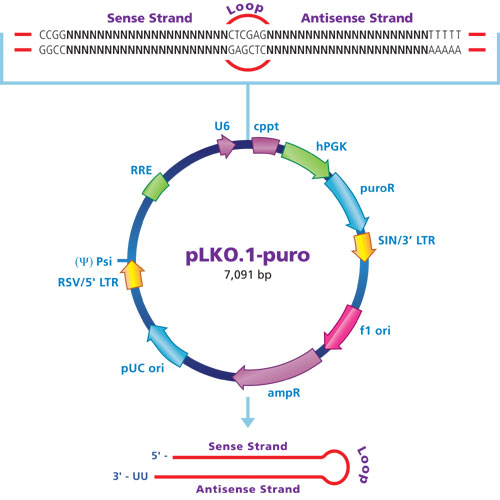
Fig.2: Silencing of the protein kinase JAK1 with Mission shRNAs.
One of the most attractive features of plasmid-based systems is the coupling of the technology to viral delivery systems. Vectors containing appropriate viral packaging signals and regulatory elements may be used to package the shRNA sequence into infectious virions. When appropriately pseudotyped, these viral particles can transduce a broader spectrum of cell lines and overcome issues faced in standard transfection methods.
Adenovirus and a number of retroviruses, such as lentivirus and murine stem cell virus are commonly used viral-delivery systems. Adenovirus utilizes receptor-mediated infection and does not integrate into the genome for stable silencing experiments, while MSCV cannot integrate into nondividing cell lines such as neurons.
The lentiviral system, pseudotyped with the VSV-G envelope protein, is an attractive system for viral packaging and delivery of shRNA constructs due to its broad tropism and nonreceptor-mediated delivery, its ability to integrate into the genome for stable gene silencing, and the fact that it does not require a mitotic event for integration into the genome. The lentiviral system is also not known to elicit immune responses, minimizing concerns of off-target effects and use in in vivo applications.
In an effort to help further the development and distribution of tools for RNAi research, Sigma-Aldrich (www.sigmaaldrich.com) joined The RNAi Consortium (TRC). The consortiums goal is to create a comprehensive library of RNAi reagents (vector-based shRNA clones) designed to knockdown expression of human and mouse genes, enabling scientists to elucidate gene function in normal physiology and disease.
Sigma-Aldrich assists the development, manufacturing, and global distribution of TRCs human and mouse lentiviral vector-based shRNA libraries, Mission shRNA.
The collection is designed and developed by the Broad Institute and is being expanded to 150,000 clones targeting 15,000 annotated human genes (Mission TRC-Hs1.0) and 15,000 annotated mouse genes (Mission TRC-Mm1.0).
Approximately 35,000 clones targeting 5,300 human and 2,200 mouse genes are currently available. The libraries include a broad range of gene families, functional classes, and druggable targets. Mission shRNA constructs are designed using a proprietary algorithm that scores potential sequences for efficient knockdown of the endogenous gene based on nucleotide content, position within the target gene, and sequence specificity via BLAST searches to minimize off-target effects.
Up to five shRNA sequences are individually cloned into pLKO.1-puro for broad coverage of each target gene and varying degrees of knockdown (Figures 2 and 3). The hairpin structure includes an intramolecular 2021bp stem and 6-base loop that is recognized and cleaved by Dicer upon expression via the U6 (pol III) promoter in the host cell. The resulting siRNA duplex then continues in the RNAi pathway by association with RISC.
The puromycin-resistance marker is present for stable selection in mammalian cells while the ampicillin-resistance marker provides for plasmid propagation in E. coli. The constructs may be used for transient or stable transfection of mammalian cells. In addition, pLKO.1-puro features allow for the generation of lentiviral particles for infection of a wide variety of cells. The 5 long terminal repeat (LTR), SIN/LTR (3 LTR), and Psi Packaging Signal permit viral packaging using 2-plasmid or 3-plasmid lentiviral packaging systems. Using this multiplasmid approach, resulting viral particles are replication incompetent and cannot be propagated, facilitating safe use of the particles.
Also, a deletion in the U3 region of the 3 LTR (SIN/LTR) does not affect generation of the viral genome during packaging but results in loss of the transcriptional capacity of the viral LTR once transferred to target cells. This feature also helps reduce the risk of emergent replication-competent viral particles and avoids problems linked to promoter interference. The integrated U6 promoter and shRNA sequence may be stably expressed within the targeted cell line.
Summary
RNA interference technology has been one of the key biological breakthroughs of the last decade and has revolutionized basic biology and gene function studies, but it also holds promise to dramatically change drug discovery and therapeutics. Over the past few years, synthetic siRNAs have delivered the genetic tools needed for studying eukaryotic systems that were previously difficult to study. However, studying systems with varying degrees of complexity encounters obstacles.
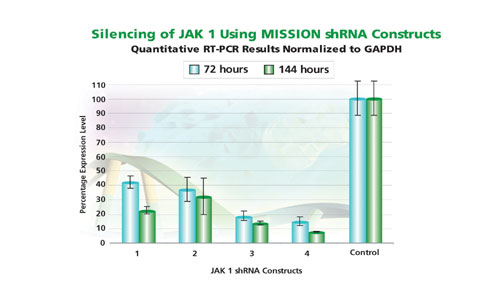
Fig.3: The lentiviral vector pLKO. 1-puro for transient transfecion and stable lentiviral expression.
Mission precloned shRNAs overcome these obstacles by providing a system for long-term silencing and phenotypic observation, plasmid for unlimited propagation, options for transient or stable transfection, and the ability to generate lentiviral particles for infection and integration of primary cells, dividing cells, and nondividing cells.