February 15, 2007 (Vol. 27, No. 4)
Combining Speed and Capacity Without Compromising Resolution
During the development and production of therapeutic proteins, characterization of structural variants that can influence safety and efficacy is a critical challenge that must be met, according to the FDA and other regulatory agencies. For instance, microheterogeneity is a major constraint in developing monoclonal antibody therapeutics. These antibody variants can result from glycosylation, oxidation, mutation, phosphorylation, amino terminal modifications, incomplete processing of the c-terminus, and asparagine deamidation.
There is a growing need for better chromatographic separation technologies to reliably resolve these variants. The technologies must combine speed and capacity without compromising resolution. Often, multidimensional chromatography is required to achieve desired separation of molecules of interest. This necessitates use of a high-capacity column in the first dimension to support sufficient sample to identify rare proteins of interest. However, high-resolution columns generally lack the necessary capacity to be considered for the first dimension.
Speed and resolution are competing factors in separations using porous media. This trade-off is exacerbated in the separation of large biomolecules such as proteins and oligonucleotides. Under these conditions, increased flow rate can lead to significant broadening of peaks due to the mass transfer problem.
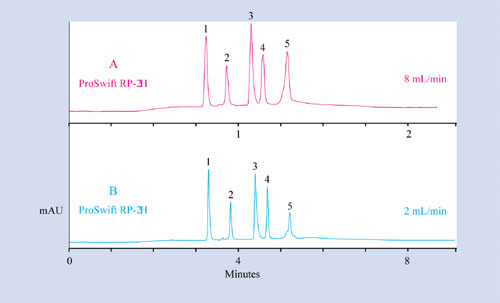
Figure 1
ProSwift Monolith Columns
Polymer monoliths, a new generation of separation media, provide a solution for this problem. Dionex’ (www.dionex.com) ProSwift™ monolith columns constitute a new family of high-resolution columns for biomolecule analysis. ProSwift columns comprise rigid, porous polymer-based monolithic columns in various formats, from nano to analytical, with diverse chemistries including reversed and ion-exchange phases. These columns feature unique monolith morphology, engineered to contain an uninterrupted, interconnected network of channels of specific, controlled size range. These large flow-through channels allow fast mass transfer even for large biomolecules.
The structure of monoliths favors high-speed separations while maintaining low back pressure (Figure 1). The capacity of monolith ion-exchange media is comparable to that of porous resins, while the resolution is similar to that of nonporous resins. The combination of those two factors gives monolithic columns better overall performance. In addition, ProSwift media exhibit stability over a wide pH range. ProSwift columns can separate and purify proteins, peptides, oligonucleotides, and other biomolecules at high-throughput for characterization studies or for LC/MS analysis.
Previously introduced monolith columns include reversed-phase columns and an anion-exchange column for separation of proteins, peptides, oligonucleotides, and other biomolecules. This tutorial demonstrates applications of two new ion-exchange monolith columns for characterization of proteins.
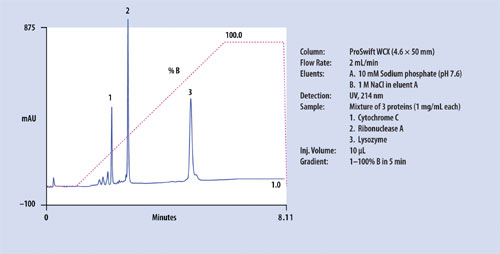
Figure 2
Separating MAb Variants
The ProSwift WCX-1 monolith column is a weak cation-exchange column with a carboxylate functional group, for separation of complex protein mixtures. Figure 2 shows a representative separation of a standard protein mixture. The ProSwift WCX-1 column is useful for separation of monoclonal antibody variants (Figure 3). Using proper gradient conditions, acidic and basic monoclonal antibody variants as well as C-terminal Lys variants can be separated. The dynamic capacity of this column is in the milligram range. The column can accept injections of up to 2–3 mg of sample, while still achieving separation of these variants in a single run.
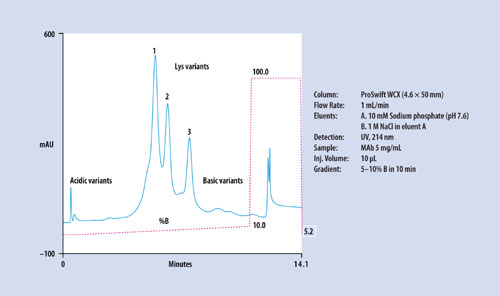
Figure 3
Separation of Complex Protein Mixtures
The second column is the ProSwift SAX-1, a strong anion-exchange column with quaternary amine functionality. Figure 4 shows an example separation of phosphorylation variants of ovalbumin. Increased loading of the sample (up to 640 µg) does not affect the separation of those variants. Up to milligram quantities of sample can be loaded for selected applications. The ProSwift SAX-1 column appears to be stable when tested over more than 800 cycles of operation.
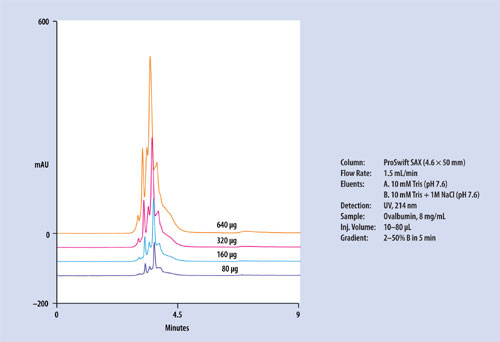
Figure 4
Conclusion
The combination of ion-exchange and reversed-phase columns is widely used for development of 2-D proteomics applications such as top-down LC/MS methods. Examples of separation techniques that require such a combination include multidimensional protein identification technology, matrix-assisted laser desorption ionization, and nanoelectrospray ionization mass spectrometry.
ProSwift WCX-1 and ProSwift SAX-1 columns expand the range of monolith columns in ion-exchange format. They exhibit all parameters needed for the above applications: pH and solvent stability, high capacity, high resolution, and high speed. These columns are optimized for protein applications and for high-speed separations in research, manufacturing, quality control, and assay development for protein therapeutics.
Srinivasa Rao, Ph.D., is technical
manager, R&D, and Nathan Bach, Ph.D., is product manager, Dionex. Web: www.dionex.com.



