August 1, 2015 (Vol. 35, No. 14)
Disease Doesn’t Have to Play as Tragedy
Molecular pathology attempts to describe and understand the origins and mechanisms of disease by evaluating the molecular content of patient samples. Increasingly, “molecular content” is being seen as an intricate, interconnected whole, a dynamic collection of interacting parts. In other words, context is all.
Context was a recurring theme at the Pathology Diagnostics Conference, a key piece of the Molecular Diagnostics Summit recently held in San Diego. At this event, several presenters emphasized the ways pathology results may become more meaningful if, for example, morphological and spatial information is preserved in molecular tests, or—more generally—imaging is integrated with data analysis.
Presenters also indicated that mining molecular pathology not only yields clinical paydirt, it also conserves scarce healthcare resources. For example, Bonnie Anderson, president and CEO of Veracyte, asked how molecular pathology could be used to impact the healthcare system and reduce costs: “Where can we impact the healthcare system to reduce cost? How can efficiency be increased by using genomic technology?” Patients, Anderson noted, often endure multiple procedures in expensive diagnostic odysseys.
A Molecular Cytology Approach to Lung Cancer
Following in the footsteps of its Afirma® assay for thyroid diagnostics, Veracyte developed Percepta®, a test for clarifying diagnosis of lung nodules. Percepta was introduced to a select market last April.
In the past, lung cancer was usually found at stage 3 or 4. When lung cancer is found so late, it is too late to attempt a surgical cure. More timely results, however, may come from new procedures, such as a screen designed for asymptomatic patients who smoke or have smoked. High-risk patients undergo a low-dose CT. If nodules are discovered, the patient undergoes a nonsurgical procedure to biopsy the nodules.
According to Anderson, “About 250,000 bronchoscopy procedures for cancer are done in a year; 40% do not yield a clear answer. That is 100,000 inconclusive procedures a year.” The next step in the diagnostic odyssey is traditionally a high-risk, high-cost surgery to reach the lung nodules directly.
To address this ambiguity in a conventional diagnostic procedure, Veracyte acquired Allegro Diagnostics, which had shown that gene expression of normal epithelial cells lining the lungs can aid in the diagnosis of lung cancer. From this information, Veracyte developed a test to determine the likelihood of cancer development based on a set of genes differentially expressed. This does not require a biopsy of the nodules, only brushing from the lining of the lungs.
According to a study published in the New England Journal of Medicine, the negative predictive value of Percepta is 94%. By offering a noninvasive diagnostic test for lung cancer, clinicians may help thousands of patients avoid unnecessary surgeries, decreasing costs and improving patient’s lives.
A Sepsis Host Response Assay
Another malady being addressed by molecular diagnostics is sepsis. Again, the problem here is the ambiguity of diagnosis. The symptoms of bacterial-induced sepsis are very similar to those induced by other causes such as burns and trauma. Conventional tests for sepsis use blood plates to screen for bacteria in the bloodstream, with a 24 hour turnaround time. Such a lengthy delay is worrisome, particularly since time is of the essence when patients display symptoms of sepsis.
Blood culture tests have high false-negative as well as high false-positive rates. High costs of treatment are also associated with sepsis; for Medicare alone, $20 billion dollars a year is spent in treatment of sepsis.
Immunexpress approaches this problem not by hunting for pathogens, but instead identifying the body’s response via gene expression in white blood cells. “We are basing the diagnosis on the host response to infection, not the infectious agent itself,” states Roslyn Brandon, Ph.D., president and CEO of Immunexpress.
Through careful studies, a set of four differentially regulated genes was defined. A good indicator of a diagnostic test’s accuracy (few false positives and few false negatives) is found in its ROC score; the numbers for SeptiCyte® range from 0.96 to 0.89.
From this information, Immunexpress developed a test that takes only four hours to perform. Having filed with the FDA in May, and pressing ahead with ongoing clinical studies, Immunexpress plans to launch SeptiCyte in the second quarter of 2016.
Dr. Brandon declares, “During clinical trials, diagnostic utility was compared to all clinical and laboratory diagnostic tools available to a clinician during the patient’s hospital stay. SeptiCyte significantly differentiated infection-positive systemic inflammation from infection-negative systemic inflammation, [performing] better than other diagnostics, both individually and in combination.”
In cancer biology, conceptual exercises have encouraged researchers to think of tumors as organs in and of themselves. Researchers interested in following this line of thought not only want to know which cells in the tumor express different markers, they also want to keep the in situ context intact. Several technical hurdles stand in the way of this ideal: antibodies developed in the same species, autofluorescence of FFPE sections, and the tedium of hand-curating multiple slides.
In Situ Analysis of Solid Tumors
Other approaches to understanding cancer are being developing in the field of tumor immunobiology. “Obtaining phenotypic information about the various immune cells that play roles in and around the tumor has been a challenge,” states James Mansfield, director of quantitative pathology applications at PerkinElmer. “Existing methods, such as fluorescence-activated cell sorting (FACS) or polymerase chain reaction assays, offer phenotypic information on homogenous samples, or single biomarkers with morphological information intact with standard immunohistochemistry.
“Blood cancers have the benefit of being amenable to FACS analysis,” continues Mansfield. “Our PHENOptics™ methodology, which includes reagents, imaging, and image analysis, enables researchers to analyze solid cancer tumors in a similar fashion while overcoming several technical hurdles.”
One component of the methodology is PerkinElmer’s Vectra® instrument, which can be programmed to remove FFPE tissue autofluorescence, correct cross-talk between fluorescent channels, quantify the per-cell marker expression, determine the cellular phenotype, and count cells. Of course, a high-resolution image of the slide is also available.
“What is truly interesting to people is not so much the staining, or even imaging and analysis, but what it does for them. It enables them to do the same kind of phenotyping and quantitation that they are currently doing in flow cytometry,” asserts Mansfield. “When cells are analyzed in situ from FFPE or frozen sections, tissue architecture is maintained, enabling spatial distance calculations between the various cell types. The capacity to acquire this information for solid cancer tumors is now finally available.”
The ability to look at multiple biomarkers in solid tumors while retaining cell location data is a recent development. As researchers determine which biomarkers are important in diagnosing and treating cancers, this instrument will be developed into a diagnostic platform. At present, it is for research purposes only.
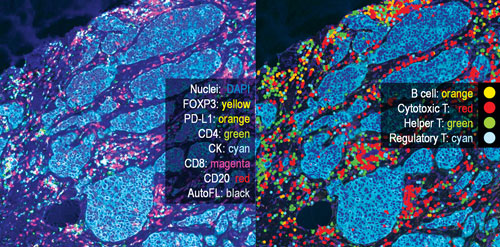
The breast cancer section shown in these images was subjected to 6-plex analysis by means of PHENOptics™, a methodology developed by PerkinElmer. Stained for FOXP3, PD-L1, CD4, CD8, CD20, cytokeratin, and DAPI, the section was imaged multispectrally on the same slice. Left: unmixed composite image with autofluorescence removed and cross-talk corrected. Right: various phenotypes of immune cells are superimposed.
Liquid Biopsies, Multimarker Panels
Historical diagnosis of cancer mutations requires a tissue sample and is based on immunohistochemical staining. Advances in technologies such as quantitative PCR (qPCR) and next-generation sequencing allow liquid biopsies from blood, urine, or other bodily fluids. The ease of this testing, with no requirement for solid tissue, offers oncologists a simple, noninvasive way to monitor disease progress and response to therapy.
“The ability to identify a particular mutation is essential in oncology, where treatment of cancer can be based on the specific mutation found in that cancer,” explains Andrew Webb, CEO of EKF Molecular Diagnostics. “Tissue samples for some tumors are difficult to obtain. Using blood as a source for tumor DNA is simple—no need for further surgical biopsies.”
EKF Molecular Diagnostics offers a system for liquid biopsy of various cancer mutations from blood samples. The source of tumor DNA is found in cell-free DNA circulating in the blood. To get enough of the mutant DNA, PointMan® PCR Primers selectively amplify the gene of interest, allowing detection of mutant DNA’s down to 0.01% or 1:10,000 copies of the mutation compared to wild type.
Wild-type DNA is not amplified in this enrichment step. The mutation in the tumor DNA that is enhanced is not specific for a particular base pair change, but will pick up any substitution at a specific local.
At present, kits are for research purposes only. Two mutations can be tested: K-ras and EGFR (other mutations are in the pipeline). Only 10 ng of total DNA is needed. Once the amplification step is complete, multimutational testing can be run by means of qPCR, pyrosequencing, or Sanger sequencing.
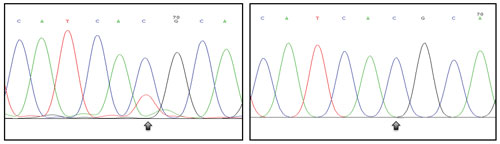
EKF Molecular Diagnostics is investigating the ability of its PointMan® kits to enrich mutant DNA from blood-based samples without the need for further biopsy. These chromatograms show PointMan-amplified cell-free DNA from patient blood (left) and nonamplified DNA (right) looking at the T790M mutation in EGFR. Arrow: position on the mutation. [EKF Molecular Diagnostics]
Data-Enriched Image Analysis
Another quest in cancer diagnostics is the integration of large quantities of visual information. Ralf Huss, M.D., chief medical officer of Definiens, states, “Pathologists use memories of images to determine what they are looking at, to diagnose.” Contextual cues aid greatly in the determination of the diagnosis.
Definiens utilizes intelligent software to analyze tissue specimens. The software processes not just imaging data, but other kinds of information, such as cancer stage, patient data (age, sex, etc.), and point in treatment—the more data the better.
The goal of this software is to combine all relevant clinical data to determine how to best treat a cancer. Ultimately, the goal is to have a product available that can be used by anyone. This algorithm will allow the determination of which drugs to use, or conversely, not to use.
“Making pathology more quantitative is foundational in converting the pathologist’s art of visually determining histomorphological spatial patterns into a mathematically solid science, by means of automated image and data analysis,” explains Dr. Huss. “This approach opens the door for a new generation of prognostic and predictive diagnostic tests for the benefit of the patient.”
This approach does for diagnostics what Google Maps does for finding directions. In Google Maps, various kinds of information—traffic information, satellite images, street views, and store information—are layered. Imagine a software tool doing the same, but for a specific tumor, based on information that had been gathered from numerous other patients. Such a tool could rely on its internal algorithms to generate “directions” to the best treatment for a specific tumor.
This approach employs mining multiparametric image analysis results for statistically significant morphological and spatial patterns. Data is then correlated with disease progression and other patient information.
The scope and complexity of this venture may seem overwhelming. But so did Google Maps just a few years ago. Someday there will be self-driving cars and cancers treated by algorithms.

Definiens, a provider of image analysis and data-mining solutions for tissue diagnostics, generated this image to represent the company’s technological approach: providing detailed cell-by-cell readouts from target structures on tissue slides, and correlating this information with data derived from other sources. This approach is meant to generate new knowledge and support better decisions in research, diagnostics, and therapy.
Companion Diagnostic Collaborations
For pharmaceutical companies seeking assistance in developing a diagnostic test to better match drugs with patients, Qiagen has several successful collaborations under its belt, including ones with Boehringer Ingelheim, Bristol-Myers Squibb, and Amgen.
The first liquid biopsy companion diagnostic with AstraZeneca for non-small cell lung cancer was launched with Qiagen’s help. A recent collaboration with Tokai Pharmaceuticals led to the development of a novel circulating tumor cell technology for separating tumor cells, then amplification of those tumor cell’s DNA.
Richard Watts, vice president for companion diagnostic partnerships at Qiagen, notes that the company collaborates with many pharmaceutical companies to develop precision diagnostic tests. “[These tests target] which patients will most benefit from their medications,” he explains. “This provides valuable insights into whether the therapy will work or not.”
According to Watts, “Qiagen offers its expertise in developing these companion diagnostics with assistance in gaining approval from the FDA with a joint submission.” Qiagen, he adds, is also “agnostic” about which platform is used to perform the diagnostic test—collaborators can use whichever one they like.
DeeAnn Visk, Ph.D. ([email protected]), is founder and principal writer for DeeAnn Visk Consulting.


