April 15, 2011 (Vol. 31, No. 8)
Cost- and Time-Effective Method Must Be Selected with Care to Ensure Accuracy
Fluorokine® Multi-Analyte Profiling (Fluorokine MAP) kits from R&D Systems are designed for the simultaneous detection and quantification of up to 100 analytes and provide a highly efficient means for profiling process-related changes in the levels of biomolecules. Measurement is achieved through a bead-based antibody-antigen sandwich method similar to that used in single analyte plate based ELISAs.
Using a Luminex®-based analyzer, beads are classified by their unique fluorescent signatures and quantified by the magnitude of the PE-derived signals that are directly proportional to the levels of bound analyte.
Many researchers have taken advantage of the efficiency and flexibility afforded by commercially available Fluorokine MAP kits. These kits offer bead sets, detection antibodies, and associated reagents for measuring the levels of cytokines, growth factors, chemokines, matrix metalloproteinases, obesity/diabetes-related factors, angiogenesis factors, and more.
Since their inception, there has been an expectation that multiplex technologies produce quantitative results similar to those of a single analyte ELISA. However, the possibility of cross-reactivity and interference multiplies with the complexity of each panel. Fluorokine MAP kits must be developed and optimized to achieve accurate and reproducible results for all analytes in the panel using a single set of multipurpose diluents.
These multipurpose diluents may not optimize any single analyte to the same degree that is possible with a unique diluent selected for a single analyte ELISA. However, the accuracy of the assay must not be compromised.
This article highlights why the results for each analyte, and the panel as a whole, should be evaluated as rigorously as a single analyte ELISA. This includes assessing specificity, recovery, and linearity of dilution, in addition to sensitivity, precision, and the ability to recognize the natural proteins. Only in this way can results that are comparable to those of a single analyte ELISA be achieved.
Variability
To investigate the potential variability in multiplex immunoassay results being published today, we evaluated several commercially available Luminex-based multi-analyte profiling kits. Each assay was performed according to the manufacturer’s respective package inserts and multiple samples were evaluated for accuracy.
Commonly used methods for evaluation of accuracy are sample linearity of dilution and recovery. Samples were serially diluted using the sample diluent specified in each vendor kit to evaluate the observed vs. the expected values (observed/expected x 100 = percent linearity).
Ideally, samples with high endogenous levels are used, but in this study the endogenous levels were too low. Therefore, serum samples were spiked with the recombinant form of the analyte (i.e., the kit standard) and diluted (Table 1A). Mean dilution recovery for each analyte in the R&D Systems kit was between 70–130%. The other three vendors had several analytes falling outside these limits (Table 1A).
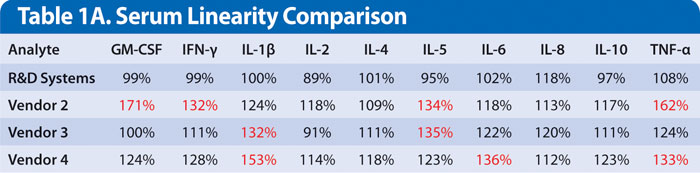
Table 1A. The serum sample was evaluated at the recommended dilution, in addition to two additional twofold serial dilutions. The result listed is the mean value for all dilution points. Values in red indicate results that are falling outside of the 70–130% performance criteria.
In addition to linearity, spike recovery can be evaluated by adding the same recombinant protein into the sample and comparing that value to a reference determined by spiking the sample diluents. All analytes in the R&D Systems kit demonstrated mean recovery between 70%–130%. As shown in Table 1B, recovery performance varied widely in kits from the other vendors (Table 1B). This lack of accuracy in sample values may increase the discrepancy in results between studies. This is especially true if the studies used kits from a variety of vendors.
Diluents used in multiplex assays must be optimized not only for accuracy, but also to minimize interferences. Heterophilic antibodies have long been a challenge in single two-site immunoassays. These antibodies can cause interference and produce error in the analytical results. This interference can be amplified when using a multiplex assay, especially in studies that evaluate samples from patients with a variety of disease states, including inflammatory and autoimmune diseases.
Such patients may have high levels of rheumatoid factor or heterophilic antibodies, particularly if they have been treated using monoclonal antibody therapy. Samples from these patients may result in false positive values unless interference is minimized.
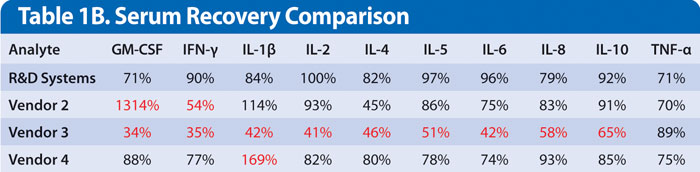
Table 1B. The serum sample was spiked with the respective vendor standard at two different levels. The result listed is the mean value for all recovery points. Values in red indicate results that are falling outside of the 70–130% performance criteria.
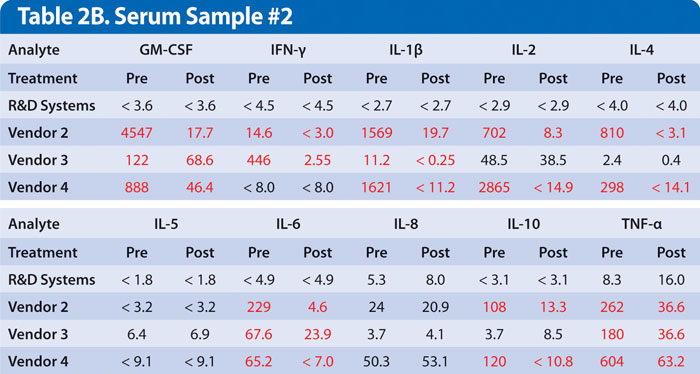
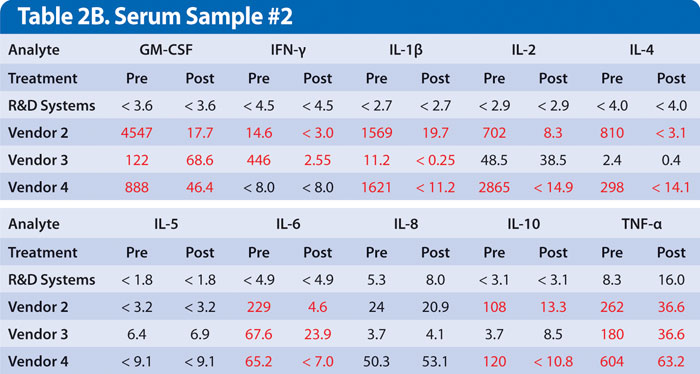
Examples of this are shown for two samples obtained from individual donors with high levels of rheumatoid factor. Samples were assayed either untreated or treated with a commercially available heterophile blocker (Table 2A and 2B). While every donor is different, multiplex kits, no less than single analyte ELISAs, must be optimized to minimize this type of interference.
Results show false positive levels for most analytes obtained using kits from vendors 2, 3, and 4, which were either partially or completely blocked by treatment with a heterophile blocker. This indicates interference in the assay that was not eliminated. A multiplex immunoassay that does not address these sources of inaccuracy will provide results that are potentially misleading or that are at odds with previously published results obtained from a single analyte ELISA or other type of quantitative assay.
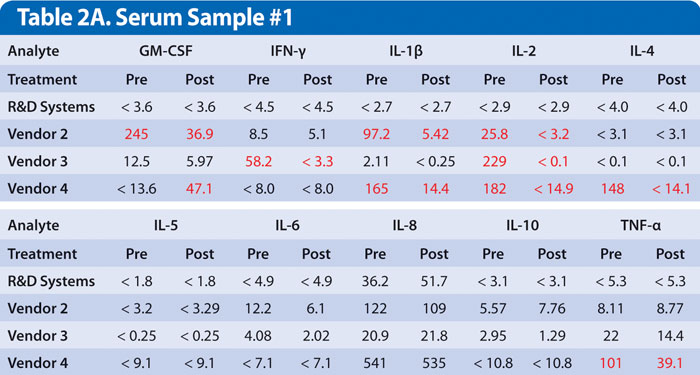
Table 2A and 2B. Serum samples from two different individuals with high rheumatoid factor were run at the vendors’ recommended dilution. Results listed as Pre were untreated. Results listed as Post were treated with heterophile blocker according to the manufacturer’s protocol. Discrepant results in red are suspected to be false positive values. Results are listed as pg/mL. Values that are less than the low standard are indicated as less than low standard value.
Conclusion
Multiplex immunoassays are a cost- and time-effective method of obtaining quantitive results using minimal sample volume. However, due diligence needs to be undertaken to ensure the accuracy of these results. With the utilization of high-quality antibodies and optimization of diluents, the user can be assured of accurate and robust results in a multiplex immunoassay using Fluorokine MAP technology.
Michael Anderson ([email protected]) is manager of new technologies, James David is team leader, new technologies, Samuel Pearlman is director of human assay development, and Jane Schmidt, Ph.D., is director of new technologies at R&D Systems.