November 15, 2016 (Vol. 36, No. 20)
Amélie Boulais Process Development Consultant Sartorius Stedim Biotech
Nick Hutchinson Ph.D. Technical Content Marketing Manager Sartorius Stedim Biotech
Fritjof Linz Ph.D. Vice President Stedim Biotech
Implementing Process-Scale Adenovirus Purification with a Single-Use Platform
The biopharmaceutical industry has tended to adopt manufacturing platforms to reduce both process development costs and the time taken for new drugs to reach the clinic. Platform processes are capable of producing many similar products from the same family with only minor adjustments to operating parameters. This allows companies to avoid having to “reinvent the wheel” for every new product that is in development while enabling them to draw upon best practices identified from previous projects.
Biopharmaceutical companies can implement recombinant viral vector platforms with a completely single-use flow path rather than installing stainless steel equipment. Installing and qualifying single-use technology is much easier, cheaper, and faster than multiple-use equipment. It allows for closed system processing that is vital when the viral vaccine is too large for sterilization by filtration.
Single-use technologies improve vaccine safety by both reducing the risk of product cross-contamination and batch-to-batch contamination. Membrane adsorbers are a single-use technology that has proven effective in virus purification. They have high capacities and give excellent yields because the macroporous structure of the membrane allows the viruses to enter the inner pore surface easily and rapidly. The virus has access to all of the ligands because of the absence of a steric exclusion effect. The design of membrane adsorbers is continuing to evolve.
Novel Membrane Chemistries
This tutorial describes the production of adenovirus with the single-use platform shown in Figure 1.
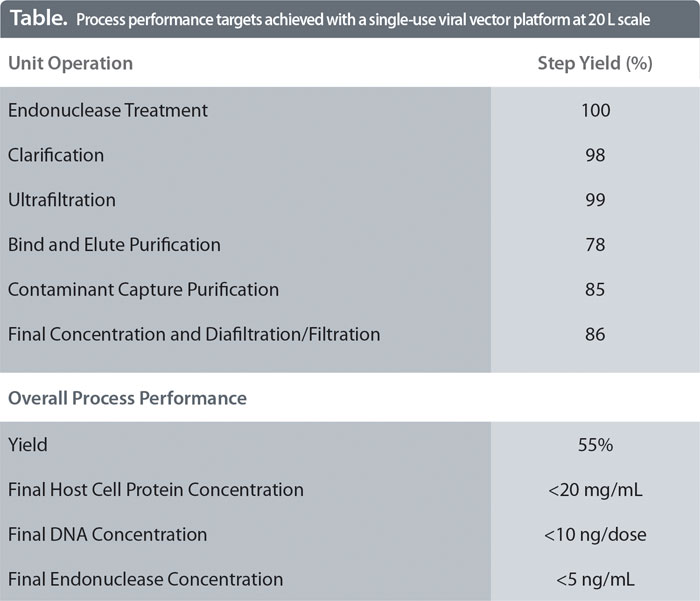
Sartorius Stedim Biotech (SSB) has assembled the platform from existing and proven technologies, available from lab to production scale, that meet the quality requirements of the vaccine industry. The approach has been successfully demonstrated for the purification of an Adenovirus serotype 5 vector, at the 20 L scale. It allows the complete purification of the vaccine in one day. Table 1 shows the performance of the platform straight-from-the-box and with minimal optimization.
The process starts in the bioreactor with the infection of HEK293 cells grown in a serum-free media. The cell concentration at infection was 1×106 cells/mL and a multiplicity of infection of five. Performing a cell lysis step at the end of the cell culture maximizes the yield of virus particles from the bioreactor but also releases large quantities of DNA. Adding an endonuclease to the bioreactor, such as DENARASE, digests the released DNA and facilitates subsequent purification operations.
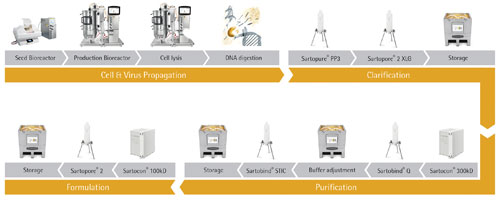
Figure 1. A single-use platform process for viral vaccine production
The next step after the DNA digest is to harvest the bioreactor. Electrostatic interactions between virus particles and diatomaceous earth depth filters can potentially reduce the step yield so, if possible, we recommend manufacturers use a polypropylene depth filter such as a Sartopure PP3 prefilter. This is constructed from a fine microfiber fleece material that improves clarification performance and has a contained capsule format.
The clarification step should be completed using a suitable sterilizing-grade filter capsule such as a Sartopore2 XLG. Filter-sizing experiments may be required at this stage of the process depending on the bioreactor biomass concentration and the amount of endonuclease added to your specific process.
SSB recommends using the cassette format for ultrafiltration steps in our downstream platform process. These allow for simple scale-up while still delivering high yields (Table).
Ultrafiltration Step
During the first ultrafiltration step, we advise manufacturers to use a membrane with a nominal molecular weight cut-off of 300 kDa. During the process run we performed, the harvested Adenovirus was concentrated 10-fold and then conditioned with 5 diafiltration volumes of 50 mM Hepes, 150 mM NaCl, pH 7.5 for loading onto the subsequent membrane adsorber step.
A 300 kDa PESU-membrane will retain virus particles but will also allow the removal of significant amounts of contaminating DNA and host cell proteins.
Figure 2 shows a chromatogram from the Sartobind Q membrane adsorber step used in the purification of the Adenovirus serotype 5 virus with a bind-and-elute method. The flow rate was limited by the standard chromatography hardware to 1MV/min.
However, the step was still performed in just 30 minutes. Under these conditions, it was possible to achieve a binding capacity of 6 × 1012 virus particles/mL of membrane and reduce the DNA level more than 15-fold.
The elution pool from the Sartobind Q membrane may require a subsequent polishing step to capture the residual nucleic acids. This step can be operated in flowthrough mode with a Sartobind STIC membrane adsorber. This is a weak anion-exchanger with a unique primary amine chemistry.
The solution must have phosphate ions added to it before it is loaded onto this impurity capture step. Process development engineers should perform an optimization study to identify the optimum phosphate concentration of the load that will allow the membrane adsorber membrane to remove nucleic acids without binding the product and reducing the yield.
In our experience with Adenovirus, a loading buffer and load with a sodium phosphate concentration of 250 mM worked well, along with a loading capacity of 6.4 × 1013 virus particles/mL of membrane. The step reduced the DNA concentration of the process stream to 0.7 ng DNA/dose, far below our target of 10 ng DNA/dose.
The Sartobind STIC elution pool can be concentrated, if required, and diafiltered with the formulation buffer in a final ultrafiltration step. For this operation, SSB recommends a membrane with a nominal molecular weight cut-off of 100 kDa.
Cassettes with stabilized cellulose membrane are extremely hydrophilic to limit fouling while maximizing the membrane flux performance and product step yield. Hydrosart cassettes were used during the Adenovirus purification.
In conclusion, a single-use platform is available for the purification of viral vaccines and SSB has demonstrated its application in an Adenovirus manufacturing process at the 20 L scale. It is flexible and can be scale-up to 2,000 L in a single-use format. The platform is a preconfigured set of unit operations for which SSB can recommend suitable operating conditions.
The platform requires little additional development work or capital expenditure but will allow drug developers to establish manufacturing processes quickly and reduce the time it takes to reach the market with new viral vaccine products.
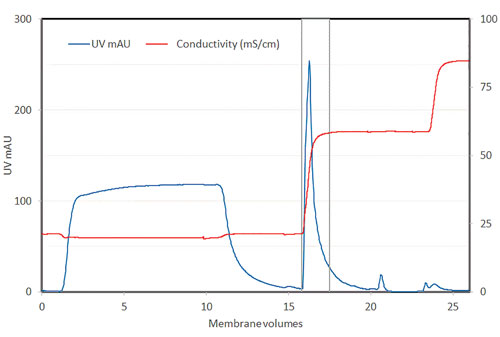
Figure 2. Typical elution chromatogram profile of Adenovirus serotype 5 using Sartobind Q membrane adsorber for primary purification step (Equilibration: 50 mM Hepes, 200 mM NaCl, pH 7.5; Wash: 50 mM Hepes, 200 mM NaCl, pH 7.5; Isocratic elution: 65% (v/v) of 50 mM Hepes pH 7.5, 1M NaCl. Strip: 50 mM Hepes, pH 7.5, 1M NaCl)
Amélie Boulais ([email protected]) is Process Development Consultant, Integrated Solutions, Dr. Nick Hutchinson works as technical content manager, and Fritjof Linz, Ph.D., is vp, purification, all at Sartorius Stedim Biotech.



