March 15, 2007 (Vol. 27, No. 6)
Jim Furey General Manager PendoTECH
Location and Small Size Can Facilitate an Accurate Reading and Unobstructed Flow Path
Pressure is an important process parameter that must be monitored in many bioprocess unit operations such as filtration, chromatography, and bioreactor production. In a multistage filtration process, such as a cell removal/clarification process from a bioreactor, pressure readings are used to measure performance of the individual filtration stages. In a tangential flow filtration process, monitoring and controlling the transmembrane pressure and the delta-pressure of the filter is necessary. In bioreactor processes, the vessel pressure needs to be controlled.
Traditionally, pressure gauges and stainless steel pressure transmitters have been used for pressure monitoring. In the spirit of the PAT initiative, electronic monitoring, recording, and trending of pressure is preferred to using pressure gauges. Even for process development, automated data collection has advantages over the use of pressure gauges for characterization of a process and record storage.
Single-use Disposable Technology
There is an increasing trend in the industry toward single-use disposable technology in process development and both clinical and commercial production. Monitoring pressure in disposable manufacturing requires optimal process solutions.
If silicone or other tubing is being used, stainless steel pressure transmitters can also be used. However, tubing adapters, gaskets, clamps, and a sanitary tee may be required with this traditional set-up. Parts cleaning, tracking, sanitization, and possible re-sterilization are also needed. If the process is sterile, a stainless steel transmitter is frequently sterilized by moist heat.
Some pressure transmitters are compatible with steam-in-place and only the product contact surface is exposed to steam, and some can be placed in an autoclave where the entire unit is exposed to steam. Many single-use process components, however, are not compatible with moist-heat sterilization temperatures and are gamma radiated before delivery to the end user. In this case, separate sterilization of the stainless steel pressure transmitter device is required, along with a potentially nonoptimal connection to the previously gamma-radiated disposable assembly.
A more ideal solution for the end user would be to have a single-use pressure sensor that arrives integrated to the single-use process assembly and gamma-radiated with the process assembly.
Disposable pressure sensors with a narrow flow-through path with luer fittings on the inlet/out, commonly used in the medical field, have been used for process R&D and small-scale production by some companies. They can be delivered to the end user as part of a disposable assembly or presterilized and individually packaged for aseptic connection to a process assembly.
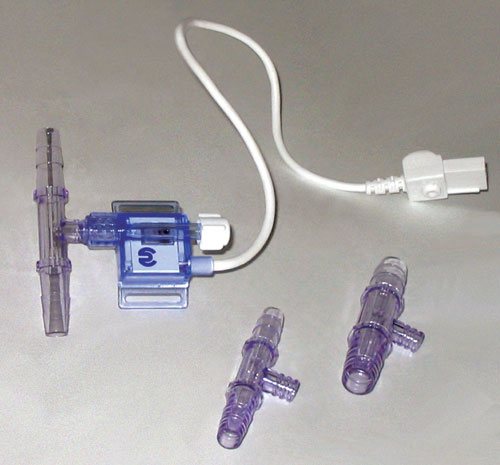
For small-scale processes, the medical sensors can be used with flexible tubing in a flow-through mode with the use of hose barb to luer adapters. When working with small ID tubing, these disposable pressure sensors are useful because it may be difficult to connect traditional pressure-measurement devices to the process stream. For wider flow paths, a hose barb fitting with a luer port can be used (Figure 1).
To better determine the applicability of MEMS disposable pressure sensors, like those used in medical applications, for use in bioprocess applications, testing was done. Micro Electro Mechanical System (MEMS) is the integration of micromachined mechanical elements, sensors, actuators, and electronics on a common silicon substrate through microfabrication technology—a system on a chip.
The MEMS pressure sensor is manufactured using a silicone micromachined piezoresistive sensing element in a Wheatstone bridge circuit where applied pressure to the circuit gives a proportional output voltage. Testing of the pressure sensors yielded positive results with the accuracy, repeatability, and robustness required for bioprocess applications.
Figure 1
Larger-scale Processes
Using these readily available medical sensors for larger-scale processes where disposable technology is increasingly being used is not optimal because of the narrow flow path and luer fitting. For larger-scale processes, flow through is not an option, and to use the sensor on a narrow dead-leg on a tee-branch of a wider ID piece of tubing may lead to undesired liquid hold-up and inaccurate pressure readings. Plus the luer fitting may loosen or become dislodged during shipping or handling of a disposable assembly that was already gamma-irradiated.
A disposable pressure sensor device optimized for larger-scale processes is now available from PendoTECH (www.pandotech.com). The sensor is embedded at the inner surface of a fitting, similar to a hose barb connector (Figure 2), and is available to accommodate from 3/8 inch to 1-inch tubing. The hose barb inlet/outlet design allows for secure connection to tubing that will not be dislodged during handling by the end-user. The location and small size of the sensor allows for an accurate pressure reading in the fluid path and also an unobstructed flow path (Figure 3). The ability to use 1-inch tubing gives a flow-rate potential of 50 L/min or more.
The pressure sensor device may also be used in a situation where a barb connector is being used, such as transitioning from one type of tubing to another. The design features no mold parting-line where the tubing is secured, which can be a source of leaks. The single barb shaft provides space for the hose to relax behind the barb, causing the tie-wrap to work like a drawstring. A tie-wrap placed over the antirotation device (designed by Eldon James) will lock the tubing, preventing it from being freed by a twisting motion.
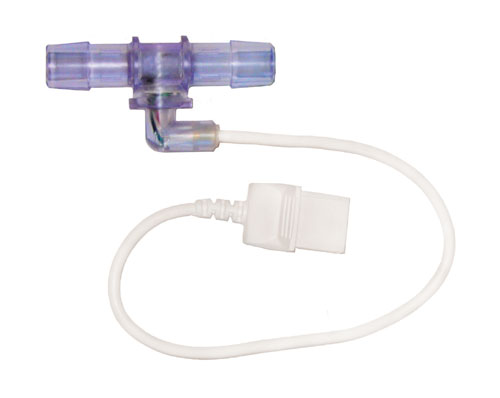
Figure 2a
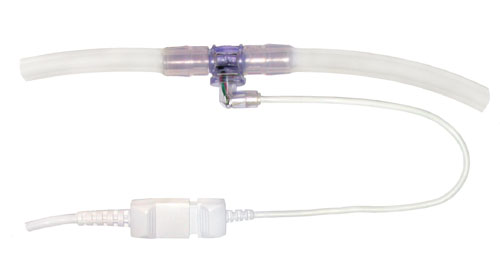
Figure 2B
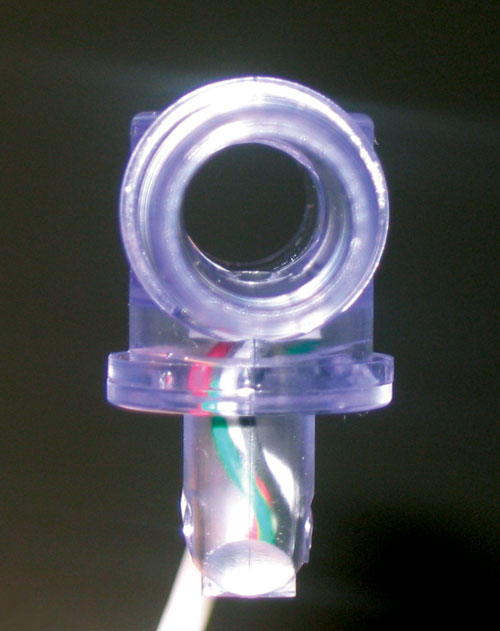
Figure 3
Design of the Sensor
The material used to mold the plastic part of the sensor is polycarbonate that meets USP Class VI tests and is compatible with gamma-radiation sterilization and ethylene oxide chemical sterilization. Before installation to the finished device, the sensor chips undergo testing, including a pressure test to over 100 psi to ensure chip integrity.
The sensors give an output of 0.646mV/psi with an applied voltage of 2.5V up to about 30 psi. From 30 to 60 psi, the sensor output is nonlinear and a polynomial calibration has been determined to account for this. Overall performance with implementation of the polynomial calibration is accuracy of better than ±0.3 psi up to 15 psi and ±2.5% of the value above 15 psi (Figure 4).
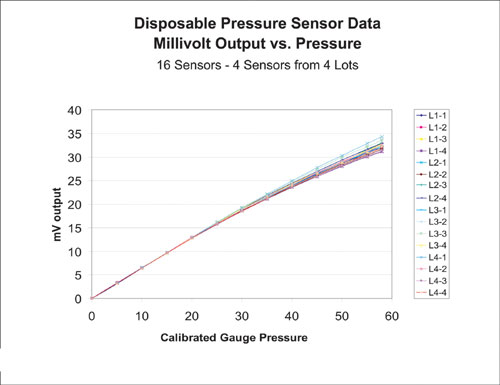
Figure 4
Process Control
The circuit in the pressure sensor chip requires a narrow range of applied voltage, and the applied voltage to the circuit gives a voltage output directly proportional to pressure. This signal is not a traditional field output signal such as 0–20 mA or 0–10 V. Therefore, to display and transmit this reading to a process control system such as a SCADA system for data acquisition/process control or a PC, an intermediate device is required.
PendoTECH has developed two systems for this purpose. The PressureMAT is configured for 1–4 disposable pressure sensor inputs. It displays the pressure, can transmit the value to both a PC (RS232 output) and a process control system (4–20mA output), and has an alarm based on user-entered min/max set-points with a dry contact relay output (Figure 5). The device is ideal for collecting data in a multistage depth filtration process (Figure 6). In addition to being used in processes for monitoring liquid pressure, the PressureMAT system has been configured for head-space pressure monitoring of glass and disposable bioreactors.
The PendoKIT custom configurable process-control system can handle up to eight pressure sensors as well as other options such as 4–20 mA outputs, alarm outputs, flow meter inputs, pinch valve outputs, scale inputs, and process analytical sensors.
The system can be configured with a flow meter and a scale input for process monitoring and data acquisition from a multistage depth filtration process. The input and outputs required to monitor and control a TFF process can be added to create a system to add process automation to a manual process (Figure 7).
The trend toward disposable manufacturing and the requirement for more on-line process monitoring combined with advances from allied technology fields to create new optimal process sensor solutions will lead to more efficient manufacturing processes.
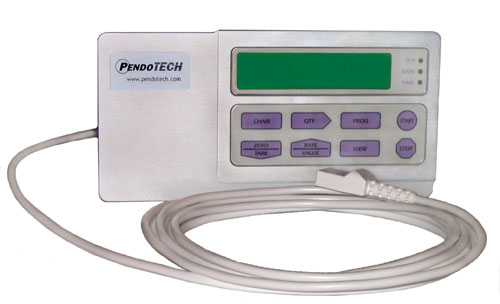
Figure 5
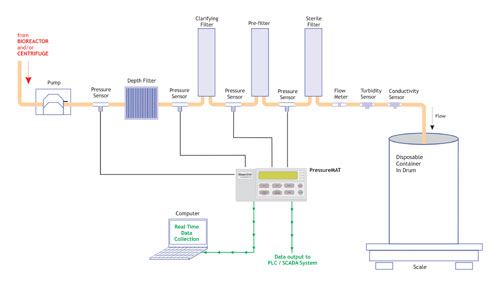
Figure 6
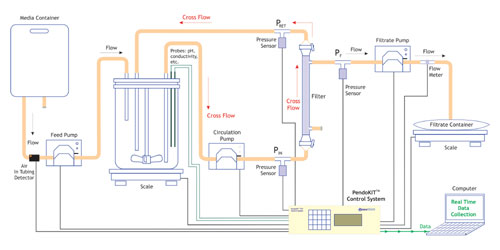
Figure 7
Jim Furey is general manager
at PendoTECH.
Web: www.pendotech.com.
Phone: (609) 802-1262.
E-mail: [email protected].